Laplace's Law Dictates the Timing of Birth, the Duration of Pregnancy, and the Mode of Delivery Through Exponential Uterine Wall Tension and Hormonal Milieu, as Well as Their Light- Dark Cycle Modulation: A Hypothesis
Ali Hegazy FRCOG*
Consultant Obstetrician and Gynaecologist, Department of Obstetrics and Gynecology, Midland Regional Hospital Mullingar, Mullingar, Co. Westmeath, Ireland
*Corresponding Author Ali Hegazy FRCOG, Consultant Obstetrician and Gynaecologist, Department of Obstetrics and Gynecology, Midland Regional Hospital Mullingar,Mullingar, Co. Westmeath, Ireland.
Received: March 05, 2025;Accepted: March 26, 2025;Published: April 28, 2025
Article Information
Citation: Ali Hegazy FRCOG. Laplace's Law Dictates the Timing of Birth, the Duration of Pregnancy, and the Mode of Delivery Through Exponential Uterine Wall Tension and Hormonal Milieu, as well as Their Light- Dark Cycle Modulation: A Hypothesis. Archives of Clinical and Medical Case Reports. 9 (2025): 71-97.
View / Download Pdf Share at FacebookAbstract
Background: The laws of physics govern the functions of human body organs. However, the law of physics that controls uterine function during pregnancy remains unknown. Spontaneous preterm labor, labor dystocia, and post-term pregnancy are significant obstetric complications, and their biological mechanisms are poorly understood. A failure to understand uterine function during pregnancy is a major shortcoming in modern healthcare.
Objective: Supporting the hypothesis, Laplace's Law dictates the timing of birth, the duration of pregnancy, and the mode of delivery through exponential uterine wall tension and hormonal milieu, as well as their light-dark cycle modulation. We will refer to this hypothesis as Hegazy's Hypothesis for Gestation (HHG).
Method: A literature search was conducted using Medical Subject Headings terms on every topic of the hypothesis in PubMed, in addition to manually searching for additional references. The scripts that were found were reviewed, examined, and condensed, along with two 40-second 3D animations.
Results: The anatomical existence of the isthmus of the cervix should be reconsidered, as there has been no convincing evidence to support its existence since Aschoff first proposed it in 1905. Uterine mechanotransduction may be the primary mechanism and system that controls uterine function during pregnancy through exponential uterine wall tension (EUWT) and the hormonal milieu. EUWT has anatomical and functional components, determinants, modulators, and a physiological mechanotransduction effect. EUWT is created and maintained by the complex interaction between the gestational sac, uterus, and cervix, whose primary function is the maintenance of EUWT. EUWT mechanotransduction with progesterone/estrogen modulation induces the stretch-dependent inhibitory uterine system (SDIUS). The SDIUS is the primary system that maintains pregnancy through autonomic intrinsic myometrial cell characteristics (AIMCC). AIMCC enables the uterus to control its function autonomously and intrinsically, due to the Myometrial Tension-Contraction Interaction (MTCI) characteristic, where high tension induces relaxation and low tension induces contraction. EUWT mechanotransduction with progesterone/estrogen modulation also induces the stimulatory system by inducing myometrial hyperplasia and hypertrophy. Embryologically and evolutionarily, the uterus is composed of two uteri. Uterine contractions (stimulatory system) create direct and indirect uterine-cervical interactions (DIDUCI). DIDUCI transforms the cervix into the lower uterine segment (LUS), through the TYVU pattern formation. Light-dark cycle modulation of the interactive inhibitory and stimulatory systems divides gestation into five clinical phases: growth, maturation, transition, parturition, and involution. Maturation phase (32-40 weeks): Nocturnally, oxytocin and melatonin modulate the stimulatory system. Additionally, cortisol modulates the inhibitory system, causing a transient nocturnal pause. The nocturnal synchronization and synergy of the two systems make the uterus an active organ at night, transforming the cervix into the LUS. This ultimately leads to nocturnal EUWT and SDUIS failure, dictating both pregnancy intervals and circadian timers. The successful transformation of the cervix into the LUS during late pregnancy is the cornerstone of pregnancy termination and achieving successful labor. The failure of this mechanism alone causes post-term pregnancy; if combined with the failure of the inhibitory system, it results in labor dystocia. A malfunction in the inhibitory system causes preterm labor.
Conclusions: Pregnancy is in a state of balance between the opposing and interactive inhibitory and stimulatory systems, secondary to EUWT mechano-transduction and progesterone/estrogen modulation. The autonomous creation, maintenance, and eventual termination of EUWT, secondary to light-dark cycle modulation, make gestation an autonomic cycle with constant intervals and circadian timers, where EUWT malfunctions alter birth timing, pregnancy duration, and mode of delivery. Laplace's law measures EUWT which may be the law of physics that govern uterine function during pregnancy.
Keywords
<p style="text-align:justify">Laplace's Law; Cervix; Parturition; Lower uterine segment; Uterine mechano-transduction; Inhibitory system; Stimulatory system; Uterine-cervical interaction; Light-dark cycle modulation; The phases of pregnancy; Interval timers; Circadian timer; Pre-term labor; Postterm pregnancy; Labor dystocia</p>
Article Details
Abbreviations: UMTHG: Uterine mechanotransduction hypothesis for gestation; EUWT: Exponential uterine wall tension; UWT: Uterine wall tension; IMCC: Intrinsic myometrial cell character; AIMCC: Autonomic Intrinsic Myometrial Cell Character; SDIUS: Stretch-Dependent Inhibitory Uterine System; IUP: Intrauterine pressure; P/E ratio: Progesterone/oestrogen ratio; LUS: Lower uterine segment; FMs: Fetal membranes; PTL: Preterm labour; IOL: Induction of labour; MTCI: Myometrial tension contraction interaction; DIDUCI: Direct and indirect uterine/cervical interaction
Background
Old traditional theories
There are three main hypotheses for the activation of the human uterus during labour: functional progesterone withdrawal, inflammatory stimulation, and oxytocin receptor activation [1]. All these hypotheses have failed to address many outstanding questions regarding physiology and gestational pathophysiology. Failure to understand properly the uterine function during pregnancy is a major shortcoming of modern healthcare, and this lack of understanding has many possible causes. Importantly, there may be a flaw in the current concept of human parturition that acts as a barrier to obtaining a better understanding of parturition. We are challenging the anatomical and physiological components on which the current human parturition hypothesis has been based since the 19th century [2]. The isthmus of the cervix does not exist embryologically [3,4], anatomically [5], histologically [6], or radiologically [7].
Introduction
Despite a considerable body of literature gathered from the few species that have been studied, the mechanisms responsible for the maintenance of pregnancy and initiation of parturition have not been fully elucidated [8]. There are two views regarding the initiation of labor. Labor is the result of the physiological release from an inhibitory effect of pregnancy on the myometrium, rather than an active process mediated by contractile agonists [9-11]. The transition from a state of uterine quiescence to the onset of contractions is the result of an increased level of contractile stimulators and a loss of mechanisms that promote relaxation [12-14]. However, the contractile stimulators and relaxation mechanisms are not well defined [12,13]. Failure to understand uterine function during pregnancy is a major shortcoming of modern healthcare systems [15]. Spontaneous preterm birth (sPTB), the leading global cause of neonatal death, affects 13 million babies annually [16], and countless efforts have failed to establish a single effective treatment for preterm labor [17]. Dystocia is associated with poor progress during labor and is a major cause of primary caesarean deliveries [18]. However, the biological mechanisms that lead to poor progress during labor are poorly understood. Inaccurate dating is the most common cause of prolonged pregnancy [19]. When a post-term pregnancy truly exists, its cause is mostly unknown. The current physiology and gestational pathophysiology do not seem far from being primitive. This study aimed to identify the systems that control uterine function during pregnancy, which may control the timing of birth and, subsequently, the duration of pregnancy and mode of delivery.
Method
A search of the literature was conducted using Medical Subject Headings terms on every topic of the hypothesis in PubMed and the MEDLINE database, in addition to manually searching for extra references. The scripts that were found were reviewed, examined, combined, and condensed, along with two 40-second 3D animations.
Laplace’s law and Uterine Mechanotransduction Hypothesis for Gestation (UMTHG)
The thesis for Laplace’s law and UMTHG
Sokolowski et al. [20] Human uterine wall tension trajectories and the onset of parturition. PLoS One. 2010;5(6):e11037 [20]. This study tested the hypothesis that high uterine wall tension might be a causal factor for preterm labor in singleton or twin pregnancies. It characterizes uterine wall tension (UWT) using ultrasound from the second trimester of pregnancy until parturition and compares preterm, term, and twin pregnancies. A total of 320 pregnant women were followed from the first antenatal visit to delivery during the period 2000-2004 at John Hunter Hospital, NSW, Australia. Uterine wall thickness, length, anterior-posterior diameter, and transverse diameter were determined by serial ultrasounds. The patients were divided into three groups: women with singleton pregnancies and spontaneous labor onset, either preterm or term, and women with twin pregnancies. Intrauterine pressure (IUP) results from previous studies were combined with our data to form trajectories for uterine wall thickness, volume, and tension for each woman using the prolate ellipsoid method, and the groups were compared at 20-, 25-, and 30-week gestation. UWT followed an exponential curve, with results increasing throughout pregnancy and that late pre-term labor (PTL) is associated with a lower UWT. For those delivering preterm, uterine wall thickness increased by P < 0.05 compared with term. For twin pregnancies, intrauterine volume was increased compared to singletons (P < 0.001), but wall thickness was not.
EUWT Components
Uterine Wall Tension (UWT) Determinants
Mechano-transduction is the process by which cells sense physical forces and translate them into biochemical and biological responses [21]. The signaling molecules involved in the mechanotransduction pathways in the human myometrium have not been extensively studied [22]. The smooth muscle of the uterus presents a unique physiological mechano-transduction mechanism during pregnancy because the tissues remodel in response to stretching imposed by the growing foetus, however, the underlying molecular and functional adaptations remain unclear [23]. We hypothesized that uterine mechano-transduction may be the main mechanism and system that controls uterine function during pregnancy through exponential uterine wall tension (EUWT), in addition to hormonal milieu. EUWT has anatomical, and functional components, and determinants, modulators, and has physiologic mechano-transduction effect. EUWT is created and maintained by the complex interaction between the gestational sac, uterus, and cervix. Uterine stretching is measured as the amount of tension exerted on the wall of the uterus. It is used interchangeably with myometrial tension and uterine wall tension (UWT) [20]. UWT is determined by uterine wall thickness, intrauterine volume, and intrauterine pressure (IUP) [24], and is measured using the Laplace equation, which applies only to a closed pressure system. The accuracy of measurements in such a system is regulated by Pascal’s law, which requires that the IUP be distributed equally within the amniotic cavity [25]. Thus, Laplace’s equation and Pascal’s law are not applicable after amniotic membrane rupture [26]. UWT follows an exponential curve, which increases throughout pregnancy, and late PTL is associated with a lower UWT [20].
UWT Modulators
A.Cervix
Cervical length is inversely related to the rate of preterm delivery in both patients presenting with symptoms of preterm labor and in asymptomatic pregnant women [27-29]. In engineering terms, an impaired cervix relates most specifically to impaired mechanical properties of the cervical stroma [30]. The strongest evidence for a primary role for impaired cervical mechanical properties is that cervical cerclage appears to benefit a select group of patients [31-33]. “The mechanical role of the cervix in pregnancy” This is the title for a study published by Myers et al. [34] in J Biomech 2015, but they didn’t explicitly define what is the mechanical role of the cervix in pregnancy. It is accepted that the structural integrity of the cervix is a key feature of normal pregnancy and abnormalities in the cervical structure are associated with spontaneous preterm birth [27,35], but the extent and the mechanism of this mechanical role have not been fully explored and understood. There is overwhelming evidence to say that pregnancy is maintained by the cervix through a mechanical mechanism where its malfunction terminates the pregnancy, but the exact nature of this mechanical role is unknown. Low UWT is associated with PTL in singleton pregnancies [20] and this leads to the question of whether there is a link between PTL due to a malfunctioning cervix and PTL due to low UWT [20]. We hypothesized that the cervix's mechanical and primary function during pregnancy is to act as a biological valve to maintain intrauterine pressure (IUP) and EUWT. (Figure 1).
B. Fetal Growth: IUP, and Uterine Volume.
The uterine volume in the twin group was reportedly larger than that in the singleton fetal group [20]. Foetal growth modulates UWT through IUP and uterine volume [36].
C. Progesterone/Estrogen (P/E) ratio: IUP and UWT
Estrogen induces the accumulation of mucopolysa ccharidesthat bind to water-answerable proteins and collagen filaments in ground substances [37]. Therefore, the accumulation of collagen and mucopolysaccharides, combined with increased water uptake after estrogen treatment, improves the plasticity of the uterine wall [38]. During late gestation in rats, estrogen ensures sufficient plasticity of the uterus to increase the conceptus size [38]. Unlike oestrogen, progesterone blocks mucopolysaccharide synthesis [37]. The low plasticity of the uterine wall observed in ovariectomized progesterone-treated rats is partly due to the action of progesterone. Estrogen secreted from the ovaries during the second half of gestation prevents IUP by improving the plasticity of the uterus [39]. Therefore, the progesterone/estrogen (P/E) ratio modulates IUP and UWT via uterine wall plasticity. The high P/E ratio decreases plasticity and increases IUP and UWT, whereas a low P/E ratio increases plasticity and decreases IUP and UWT.
D. P/E Ratio and Uterine Volume.
Progesterone, estrogen [40], and uterine wall stretching induce myometrial hyperplasia and hypertrophy, which causes an increase in uterine volume. Uterine wall tension modulators include the cervix, fetal growth, and P/E ratio.
E. Summary of UWT Modulators
UWT determinants include uterine volume, IUP, and uterine wall thickness, whereas UWT modulators include fetal growth, P/E ratio, and cervix. Each factor modulates UWT through dual mechanisms, IUP, and uterine volume modulation. (Figure 2) Meanwhile, the thickness of the uterine wall did not change during pregnancy [20].
Anatomical and Physiological EUWT Components:
- • The fetus/fetuses
- • Placenta
- • P/E ratio
- • Amniotic Fluid
- • Fetal Membranes
- • The Cervix
- • Fetal fibronectin.
These components are responsible for the development of the physiological function of EUWT, and malfunction of any component may cause EUWT failure.
Autonomic intrinsic myometrial cell character (AIMCC)
How does the uterus control its contractions during pregnancy and labor?
• Uterine innervation during pregnancy:
The uterus comprises myogenic smooth muscle, which contracts without neuronal or hormonal inputs [42]. In contrast to vascular smooth muscles, myometrial cells in smooth muscles are sparsely innervated and become less innervated during pregnancy [43,44]. The uterus essentially becomes denervated during gestation [45]; therefore, it is unlikely that coordinated nervous regulation of the myometrium is centrally orchestrated [46].
• The autonomous control of myometrial cells:
In vitro studies have shown that quiescent myometrium from term uteri, and when placed in an isotonic solution, contracts vigorously and spontaneously without added stimuli [9]. Hurd et al. [47] determined whether myometrial strip shortening alters spontaneous contractility in myometrial strips obtained from pregnant women at term. They found that contractility increased by 29% and 34% in strips shortened by 4% and 6%, respectively, according to a mechanism that involves prostaglandins [47]. In myometrium strips obtained from women at term, stretching increases the inhibitory prostaglandin level, and shortening increases the stimulatory prostaglandin level, which suggests that prostacyclin synthase and prostaglandin F2 alpha synthase might be activated differentially in a single-cell type [48]. The regulation of the uterine contractile mechanism depends largely on humoral factors, intrinsic factors within the myometrial cells, or both What are the intrinsic factors within the myometrial cells that control their function?
• Myometrial tension/contraction interaction (MTCI) hypothesis:
The current understanding of the myometrial contraction cycle is called the contraction-tension relationship. The change in uterine tension occurs secondary to contraction and relaxation. Uterine contraction increases myometrial tension, while relaxation decreases myometrial tension. We have another proposal and explanation for myometrial contraction tension relationship. Myometrial cells autonomously and intrinsically contract and relax, secondary to myometrial tension changes, wherein high tension induces relaxation and low tension induces contraction [20,42,47,48]. The myometrial cells inherently contract [42], and we hypothesize that it intrinsically relaxes through the myometrial tension contraction interaction characteristic (MTCI). We hypothesize that myometrial cells are like a biological spring where they inherently contract and relax secondary to tension changes.
MTCI may be the link among all the aforementioned evidence-based studies. MTCI may be the autonomic intrinsic myometrial cell character (AIMCC) that enables the uterus to control its function autonomically and intrinsically secondary to changes in tension, where high tension induces relaxation and low tension induces contraction. (Figure 3) (AIMCC = IMCC)
Fail-Safe Stretch-dependent Inhibitory Uterine System (SDIUS)
Prostaglandin I2 (PGI2) is a potent, smooth muscle relaxant whose concentration increases [49] in maternal plasma [50] in urine [51] and myometrium [52] as pregnancy progresses until labour begins. Myometrial stretching increases PGI2 production [53]. In a rat model, myometrium stretched by the conceptus exhibited increased PGI2 production [54]. Additionally, myometrial stretching increases the production of prostaglandin E2 (PGE2) and PGI2 metabolites and decreases the production of PGF2-alpha metabolites [53]. Because PGI2 is a potent relaxant of the uterine myometrium [55], increased PGI2 production may help to maintain uterine quiescence. Stretch-related induction of PGI2 production could be an essential protective mechanism to decrease the risk of premature labour [47]. This “stretched” state is similar to the “resting” length of the myometrium at term before labour, which is always tension caused by the intrauterine contents [48]. Increased stretch-dependent K+ channels stabilize membrane potential and control contraction during pregnancy; downregulation of these channels induces the onset of delivery [56]. We hypothesized that there is a stretch-dependent (mechano-transduction) inhibitory mechanism; which is the main mechanism that maintains pregnancy through AIMCC. This “stretched” state approximates the “resting” length of the myometrium at term before labor, which is constantly under tension as a result of the intrauterine contents [48]. According to UMTHG, malfunction of any component anatomical or physiological components of EUWT will cause dysfunction of the stretch-dependent inhibitory uterine system, resulting in termination of pregnancy.
Direct uterine inhibitory system: Progesterone, corticotropin-releasing hormone [57], and nitric oxide [58] are direct myometrial relaxants. Studies in animals [59-62] and humans [59,61-63] have shown that nitric oxide may be an important mediator of uterine quiescence and cervical competence before labor.
Stimulatory System
1. Changes in the lower uterine pole:
• Radiological Changes of the Cervix.
Studies on parturition in animals suggest that preparation for labor is evident at relatively early stages of gestation [40,64]. Ultrasound has demonstrated that the cervical function is “continuous” rather than binary [65]. The cervix shortens in the third trimester [66-68], The functional cervical internal os moves down, and the cervical funneling depth opens directly into the LUS [69]. The cervical gland area detection rate significantly decreased after 32 weeks of gestation, and the cervical length and cervical gland area detection rate changed simultaneously [70]. The endocervical gland's progressive absence is associated with progressive shortening of the cervix in the third trimester [70]. Magnetic resonance imagining (MRI) study was conducted to compare cervical anatomy in the second and third trimesters. The third trimester is associated with significant descent of the amniotic sac. Descent of the amniotic sac is associated with modified anatomy of the uterocervical junction. Taken together, these 3D changes are associated with a shorter cervix in the third trimester [71].
• Changes in the FMs.
Separation and decreased adherence between the amnion and choriodecidua occur as gestation progresses, and term human FMs have a weak zone overlying the lower uterine pole and cervix before the onset of labor [72].
• Changes in Cervical Collagen.
Biochemical changes associated with cervical softening include a decrease in collagen concentration by nearly 50%, an increase in collagen solubility, a decrease in decorin concentration, and an increase in hydration compared with the respective values in non-pregnant women [73]. Term pregnancy is associated with a 40–50% decrease in the decorin concentration, which is postulated to decrease the stability of the collagen network [73]. Where does the cervical collagen go? Where does cervical collagen disappear?
• Clinical Changes in the Cervix.
Cervical remodeling can be loosely classified into four distinct but overlapping phases: softening, ripening, dilation, and postpartum repair [74]. Cervical effacement begins at approximately 32 weeks for term birth and as early as 16–24 weeks for preterm birth [75].
• Radiological features of the Cervix deformation.
Sonographic studies of the natural history of cervical deformation associated with insufficiency revealed that the cervix deforms in a ‘TYVU’ pattern [76,77] a closed cervix corresponds to the ‘T,’ progressive funneling corresponds to ‘Y’ and ‘V,’ and end-stage funneling corresponds to ‘U.’ Zilianti et al. [78] described the appearance of cervical effacement as observed by transvaginal sonography as a progression through the letters T, Y, V, and U. Their concept is depicted in modified form by Iams [75], where a closed cervix corresponds to ‘T,’ progressive funneling corresponds to ‘Y’ and ‘V,’ and end-stage funneling corresponds to ‘U.’
• Viscoelastic Characteristics of the Cervix and FMs.
Foetal membranes (FMs) and the cervix have viscoelastic characteristics [72]. The viscoelastic material has an elastic component (collagen) and a viscous macromolecule (proteoglycans) [79]. The viscoelastic properties include creep (a time increase in deformation with a constant load), stress relaxation, and non-recoverable deformation [72].
Transformation of the cervix into the LUS through the TYVU pattern formation might be the link to the aforementioned evidence-based studies. This hypothesis dates back to as early as the 1880s, by Roederer and Mauriceau who stated that the LUS was derived from the cervix [2]. If this view is accurate and the LUS is derived from the cervix, it could be argued that the cervical functions will never be discovered as long as the isthmus hypothesis persists.
• Unanswered Questions:
A. What is the uterine force that transforms the cervix into the LUS?
B. What is the mechanism that transforms the caudal cervix into the cranial LUS?
C. Why does the cervix lose strength and deform in the radiological YVU pattern?
D. When does the cervix transform into the LUS?
E. What is the effect of cervical transformation into the LUS?
2. Development of the stimulatory system
The two early distinct phases of myometrial growth from early to late pregnancy are myocyte hyperplasia in the first half of gestation (“proliferative” phase) and myometrial hypertrophy associated with an increase in the smooth muscle cell size (“synthetic” phase) in the second half of gestation
This indirectly indicates that mechanical stretching exerted by the growing fetus may be one of the main signals that cause the uterine myocytes to shift from the proliferative phase to the synthetic phase of myometrial growth [41], when EUWT is ascending to the peak [20]. The third phase is the acquisition of uterine contractions in the third trimester [80].
3. Complex Uterine System
Embryologically and evolutionarily, the human uterus is composed of two separate organs: the paramesonephric endometrial–subendometrial unit and the outer nonparame sonephric myometrium [81-84]. Histologically, it consists of three layers arranged in different directions, with a specific and different outcome caused by the contraction of each layer. The cervix comprises three collagen layers [7]. Functionally, the uterus is composed of two different organs, the corpus and the cervix, wherein one contracts and the other dilates [85].
4. Cervical-uterine Interaction
The implication for spontaneous labor is that two systems, one controlling cervical compliance and the other myometrial activity, must be coordinated for successful, low-risk labor; one system functioning without the other will not result in progressive labor [85]. The relationship between uterine contraction, cervical dilatation, and head descent is mathematical [86,87]. How are these systems coordinated? The physical process of cervical deformation and its relationship to cervical-uterine interactions are poorly understood [88]. What is lacking is an understanding of the physical process of cervical deformation as it relates to the interaction of the cervix and the uterus [88]. Objective investigation of the connection between the uterus and the cervix is a promising area of study [89].
5. Uterine-cervical Interaction and the YVU pattern formation
The cervix deforms and effaces in the TYVU pattern in preterm labor [76] or full-term labor as well [78]. This The cervix loses strength through the TYVU pattern formation either in preterm labor or full-term labor to be transformed into the LUS. This means the central cervical collagen is stretched upward and disappears progressively from the cervix's center, and it reverses instantly back after fetal delivery. How and why the cervix deforms in TYVU pattern formation?
The embryologically and evolutionarily complex uterine system:
• Two uteri with three myometrial uterine layers are arranged in different directions, and their contractions create forces in various directions.
• The uterus is fixed through the cervix to the pelvic bone by strong uterine and cervical ligaments.
• The cervix consists of three collagen layers, where the most central layer is longitudinal and less fixed by the uterine and cervical ligaments.
• The cervix is laterally fixed by strong ligaments at a right angle relative to the direction of the upward uterine traction force.
• The fetus indirectly connects between the uterus and the cervix.
Functionally:
a) For the uterus to push the fetal resistance, the contraction of the outer uterine layer (the outer uterus) fixes the whole uterus into the pelvic bone through strong cervical and uterine ligaments. causing an initial cervical stretch in between the uterus and strong uterine ligaments, resulting in direct uterine-cervical interaction.
b) Contractions of the evolutionary layer will decrease all the diameters of the uterine cavity, creating two opposite forces:
• The first force pushes the fetus through the pelvic inlet and then the cervix, resulting in cervical deformation through indirect uterine-cervical interaction.
• The second force simultaneously stretches the inner cervical collagen layer upward, causing also direct uterine-cervical interaction.
c) The inner collagen layer, which is less fixed, will be the most stressed and upward-stretched layer.
d) The outcome of the contractions of the complex uterine system is direct and indirect cervical interaction (DIDUCI) resulting in the cervical YVU pattern formation. Animation 1 and Figure 4.
Animation 1:
Animation for the hypothesis that the cervix transforms into the LUS through the TYVU pattern and inverted U pattern formations due to direct and indirect uterine cervical interactions.
https://www.youtube.com/watch?v=_VlaC76kbv8&t=159s
This complex DIDUCI interaction deforms the cervix into a TYVU pattern formation, where the more central the structure is to the cervical canal, the earlier and more severe the response to the stress force will be. The cervical glands (more central) progressively disappeared 2 weeks before cervical shortening [70]. Anatomically, the cervix lies below the pelvic inlet, and we would argue that the success and efficiency of DIDUCI in deforming the cervix depends on the easy crossing of the fetal head through the pelvic inlet, where any cephalo-pelvic disproportion will cause DIDUCI malfunction. In summary, the outer part of the cervix is well fixed and less affected, while the more central area, which is less fixed, is deformed by a stronger force, the evolutionary layer. Unequal fixation of the cervix with unequal force leads to an unequal response, resulting in cervical YVU pattern formation.
Studies are needed to understand how these uterine layers coordinate together to deform the cervix through the TYVU pattern formation, which would help obstetricians and scientists to treat the malfunction of the inhibitory and stimulatory systems.
6. Cervical length at 37 weeks versus 30 weeks
Andersen et al. [90] reported the cervical length to be 40– 44 mm in the 30th gestational week. The cervix shortens in the third trimester; Ramanathan et al. [68] measured the cervical length at 37 weeks and classified individuals into five groups according to the cervical lengths of 1-10, 10-20, 21-30, 31-40 and 41-50 mm. Based on studies matching the cervical length in pregnancy with the criteria of cervical funneling [69,75,91] and the cervical length observed in the Ramanathan et al. study groups, and according to UMTHG, we would argue the following:
• The onset of the transformation of the cervix into the LUS starts from 30-32 weeks.
• Cervical shortening in the third trimester is due to its transformation into the LUS.
• The variation in cervical length at week 37 of gestation in Ramanathan’s groups indicates that:
a) Each group shows a different degree of transformation into the LUS.
b) Each group matches a different TYVU pattern formation.
c) The difference in cervical length at 37 weeks' gestation compared to 30 weeks is related to the efficiency of the system that transforms the cervix into the LUS. This varies from full success in the group of patients with a cervical length of 1-10 mm to complete failure in the group of 41-50 mm.
d) Failure of EUWT is imminent in the group of patients with 1-10 mm cervical length.
• The cervix contributes to the uterine volume when it transforms into the LUS, in addition to maintaining IUP.
• The urinary bladder has no direct anatomical relationship to the cervix because the cervix transforms into the LUS and reverts instantly after delivery.
There is overwhelming evidence that the cervix deforms in an explicit geometric deformation through TYVU pattern formation. We would argue this is the mechanism of transformation of the cervix into the LUS, secondary to DIDUCI, and it is a prerequisite for successful termination of pregnancy and initiation of labor.
The Interactive Forces, Mechanisms, Systems, and Organs of Pregnancy
In summary, there is evidence that pregnancy is a state of balance between opposing and interactive forces, mechanisms, systems, and organs secondary to EUWT mechano-transduction and progesterone/estrogen modulation:
• Opposing Forces: The force from the natural gestational sac and fetal growth which creates and maintains EUWT against the force from uterine contractions.
• Opposing Mechanisms: Myometrial stretching due to EUWT against myometrial shortening from uterine contractions.
• Opposing Systems: The stretch-dependent inhibitory system secondary to EUWT maintains pregnancy, and the stimulatory system weakens the cervix through the YVU pattern formation, leading to EUWT failure.
• One Origin: EUWT mechano-transduction and progesterone/estrogen stimulation directly induce the stretch-dependent inhibitory system and indirectly induce the stimulatory system by causing myometrial hyperplasia and hypertrophy.
• Bi-directional interactive uterine cervical modulation: biomechanical against Biophysical:
a) A biomechanical system (DIDUCI) consisting of the uterine corpus and the GS opposes a biophysical system (EWUT) led by the GS and the cervix.
b) The cervix inhibits uterine contractions by maintaining EUWT, while uterine contractions weaken the cervix and transform it into a birth canal through YVU pattern formation. (Figure 5)
Pregnancy Phases or Phases of Uterine Activity
There is a significant difference between the current hypothesis and our hypothesis for the pregnancy phase or phases of uterine activity regarding the components, mechanism, and outcomes.
• Current hypothesis
To consider how the uterine activity is regulated during the latter part of pregnancy and labor, four distinct physiologic phases are described [92]. This concept was also adopted by William’s obstetrics regarding uterine activity during pregnancy. The regulation of uterine activity during pregnancy and labor can be divided into four distinct physiologic phases, which include the following: phase 1, uterine quiescence, and cervical competence. Phase 2, uterine activation and cervical ripening, phase 3, uterine stimulation, and phase 4, involution [93,94].
• UMTHG
The clock that measures the duration of pregnancy consists of two interacting timers: an interval timer measuring the overall length of gestation and a circadian timer that defines when a 24-hour cycle birth occurs [95]. The timing of birth dictates both pregnancy intervals and circadian timers. The pregnancy interval timer is independent of the suprachiasmatic nucleus, since animals with lesioned suprachiasmatic nuclei deliver at an appropriate gestation duration on average [96]. However, the circadian timing of birth depends on the hypothalamic suprachiasmatic nucleus, as demonstrated by lesion studies in rodents [97]. Although the onset of spontaneous human parturition preferentially occurs during the nighttime and early morning hours, no convincing physiological explanation has been proposed for this pattern [98]. Maintaining appropriate circadian phases requires entraining cues (zeitgebers) that are likely to be neural, endocrine, metabolic, or a combination thereof. For many peripheral clocks, the neuroendocrine output cues of melatonin and glucocorticoids play vital roles [99]. Indeed, these two hormones significantly affect the endogenous circadian clock in various peripheral tissues [100-102]. We propose that light-dark cycle modulation of the inhibitory and stimulatory systems divides gestation into five clinical phases: growth, maturation, transition, parturition, and involution. Animation 2 Figure 6.
The clinical phases of pregnancy
A. Growth Phase
The growth phase is from conception until 30 weeks, during which the inhibitory system is dominant, and the foetus grows [103]. UWT increases [20], the uterus hypertrophies and strengthens [40], and the cervix is competent, with no change in its length [90] and poorly coordinated contractures or Braxton–Hicks contractions [104].
B. Maturation Phase and The Timing of Birth
1. Transient Nocturnal Pause of the Inhibitory System:
The timing of birth controls both the pregnancy interval and circadian timers. During the later stages of human pregnancy (approximately 34–35 weeks), the placental barrier to maternal cortisol weakens, at least partially [105], which causes maternal cortisol to modulate foetal endocrinology. There is an inverse relationship between maternal cortisol and estriol levels at 30–31 and 34–35 weeks, which is consistent with the maternal effect observed on fetal adrenal function [106]. A decrease in maternal cortisol levels at night results in a surge in estradiol secretion [106], decreasing the P/E ratio. (Figure 7) The decreased P/E ratio increases uterine wall plasticity [38], which decreases IUP and EUWT, and this temporarily releases the uterus from the inhibitory effect of the stretch-dependent inhibitory system. Therefore, we would argue that the inhibitory system temporarily wears off nocturnally in the third trimester, secondary to nocturnal cortisol modulation. Also, this progesterone: estrogen ratio switch leads to uterine prostaglandin production and labor Cortisol modulates the inhibitory system, causing a transient nocturnal pause.
2. Nocturnal Activation of the Stimulatory System
Continuous monitoring of normal uterine contractile activity during late-term pregnancy in humans has shown an increased frequency between 2030 and 0200 h [108]. During the third trimester, the uterus becomes a biomechanically active organ [80] because of the modulation of melatonin [109] and oxytocin [110].
3. Nocturnal Synchronization of the Two Systems
Oxytocin and melatonin modulate the stimulatory system, while cortisol modulates the inhibitory system. (Figure 8) Nocturnal synchronization and synergy of the two systems occur. The uterus is biomechanically active at night and in the early morning [80,108]. The nocturnal elevation of maternal plasma oxytocin and estradiol concentrations correlate with circadian uterine activity and the suppression of estrogen production in these animals prevents an increase in nocturnal uterine activity [111]. In Rhesus monkeys and baboons, parturition can last several days. Synchronized uterine contractions occur each night and disappear during the day until delivery [112,113]. Oxytocin and melatonin modulate the stimulatory system. Additionally, cortisol modulates the inhibitory system, causing a transient nocturnal pause. The synchronization and synergy of the two systems make the uterus an active organ at night. Figure 8.
4. Effect of Nocturnal Synchronization of the Two Systems
In the 30th week of gestation Andersen et al. reported a cervical length of 40–44 mm [90], whereas Ramanathan et al. reported the cervical length at 37 weeks [68]. We noticed that the cervix was progressively shortened during the third trimester. Based on studies matching the cervical length in pregnancy with the criteria of cervical funnelling [69,75,91], and the cervical length observed in the study by Ramanathan et al. [68] group with a cervical length of 1–10 mm, according to our hypothesis, represents:
• The cervical length at full term.
• The cervix loses its strength completely.
• The cervix is fully effaced.
• Impending EUWT failure.
• The cervix keeps its strength to the last fibers, up to 1 mm.
• A cervical length of 1-10 mm is equal to that of the U pattern in the TYVU pattern.
• The last deformed cervical fiber is the lowest, that is, the U pattern.
• The U pattern-initiated labor as 100% of the patients delivered within 2 weeks.
• According to our hypothesis:
a) The full success of the mechanism that transforms the cervix into the LUS.
b) The group of patients with cervical length 41–50 mm at 37 weeks is equal to the T pattern, and 0% of patients delivered at 40 weeks, which represents the complete malfunction of the mechanism that transforms the cervix into the LUS.
So, here is a strong relationship between the cervical length at full term and the onset of labor.
Eventually, by the end of the maturation phase, the group of patients in whom the cervix completely loses its strength signals EUWT failure nocturnally and initiates parturition. So, there is a strong relation between a complete loss of cervical strength, EUWT failure, and the onset of labor. In conclusion, oxytocin and melatonin modulate the stimulatory system. Additionally, cortisol modulates the inhibitory system, causing a transient nocturnal pause. The synchronization and synergy of the two systems make the uterus an active organ at night, transforming the cervix into the LUS, resulting in nocturnal EUWT failure dictating both pregnancy intervals and circadian timers.
5. Preterm labor is also Circadian
Preterm birth most commonly begins between midnight and 0600 hours [114]. The periodicity of the onset of birth in multiple pregnancies demonstrates a rhythm similar to that of singleton pregnancies, where labor most commonly begins between midnight and 0800 hours [115]. In this hypothesis, EUWT with light-dark cycle modulation is the mechanism of pregnancy maintenance and termination, with no differentiation between full-term or preterm pregnancy or labor.
6. Timing of Birth in Other Species
The timing of birth in other species, such as sheep and mice, is predictable, and underlying regulatory signals have been defined [116]. In sheep, Liggins (1974) [117] showed that parturition is initiated by increased activity of the fetal hypothalamic-pituitary-adrenal axis to produce a surge of cortisol that decreases progesterone production by the placenta, which initiates parturition. In mice, parturition is initiated after the upregulation of endometrial cyclooxygenase, which PTGS1 encodes and subsequently generates prostaglandin F2α (PGF2α) [118-120]. PGF2α from the endometrium acts on the corpus luteum of the murine ovary to induce luteolysis, decrease serum progesterone, and induce contractile proteins in the uterine myometrium. Parturition lasts for several days in rhesus monkeys and baboons. Synchronized uterine contractions occur each night and disappear during the day until delivery [112-113].
While in humans:
• There are three main hypotheses for the activation of the human uterus during labor: functional progesterone withdrawal, inflammatory stimulation, and oxytocin receptor activation [1].
• Liggins et al. showed that the specific pathways operating in sheep do not operate in the same way in humans [121].
• A decrease in circulating progesterone levels does not occur in humans during full-term or preterm labor [93].
• Cortisol plays a key role in promoting fetal organ maturation in humans; however, parturition in humans is not initiated by fetal hypothalamic-pituitary-adrenal activity [116].
• Regarding number c and d, we showed that cortisol plays a vital role in the initiation of labor through the modulation of the P/E ratio.
C. Transition Phase
An example of this phase is patients with a cervical length of 1–10 mm at 37 weeks, as was seen in the study by Ramanathan et al. [68] with a 100% delivery rate within 2 weeks. The stimulatory system fully matures after the cervix successfully transforms into the LUS. The inhibitory system wears off as foetal growth plateaus [103] the cervical resistance reaches zero, or the FMs have ruptured. Therefore, the uterus is no longer a closed space, Laplace’s law and Pascal’s principle are no longer applicable, EUWT fails, and it can no longer stretch and inhibit myometrial cells. In the study by Krispin [122], half of the women with premature rupture of membranes at term, who were managed expectantly, delivered within 33 h, and 95% delivered within 94–107 h of membrane rupture. The transition phase is the period between the failure of the pregnancy maintenance mechanism (EUWT failure) and the onset of labor, which varies from a few hours to a few days. Does the transition phase in this hypothesis equal Friedman's latent phase of labor?
Animation 2:
Animation for the hypothesis, that Laplace's Law dictates the timing of birth and mode of delivery through exponential uterine wall tension and hormonal milieu and its light-dark cycle modulation. https://www.youtube.com/ watch?v=YxIt2WO0NXY
Evidence-based Support for Laplace’s Law Hypothesis for Gestation
In 2010, Sokolowski et al. [20] published an impressive study on the changes in UWT during pregnancy. Their study tested the hypothesis that high uterine tension may be a causal factor for PTL in singleton or twin pregnancies. The outcome of this study refutes the hypothesis and suggests the opposite. Sokolowski et al. showed that UWT is exponential throughout pregnancy and that late PTL is associated with a lower UWT.
We will discuss the work of Sokolowski et al. with our hypothesis at three points:
1) We consider Sokolowski et al.’s the thesis for our hypothesis:
• EUWT is the biomechanical force that maintains the inhibitory system.
• Low EUWT caused inhibitory system failure and terminated the pregnancy.
2) Sokolowski et al. [20]: comparison between the findings in the singleton full-term and twin groups
• Myometrial tension in twin gestations was not increased relative to term singleton gestations.
• The uterine volume was considerably larger in the twin group, despite the exponential UWT.
• The twin group has delivered prematurely.
Comment according to our hypothesis: The cause of preterm birth in multiple gestations or polyhydramnios is possibly increased uterine distension, which leads to increased uterine contractility [123]. The increased uterine volume might increase the uterine force, which induces premature transformation of the cervix into the LUS with premature EUWT failure. Despite the difference in delivery time, UWT was exponential in both groups, as the competent cervix maintained its function in the last fiber [68].
3). Sokolowski et al. [20] found that the singleton preterm group, associated with pregnancies of late preterm birth, has the following characteristics:
• A lower UWT that became significant by week 30.
• Slightly greater uterine volumes than the singleton full-term group.
• The data showed a small but significant increase in uterine wall thickness in women who delivered preterm infants with a singleton gestation.
Comment according to UMTHG: Low UWT in the preterm group may be due to one or more of the following reasons:
a) The cervix may be less competent at maintaining IUP, which leads to a decreased IUP and low UWT.
b) Our hypothesis showed that the cervix modulates EUWT through a dual mechanism, maintains IUP, and contributes to the uterine volume when it transforms into the LUS. The cervix may contribute prematurely to uterine volume due to its premature transformation into the LUS, which leads to decreased UWT.
Summary, we would consider Sokolowski et al.'s study [20] is the thesis for our hypothesis.
Clinical Support for Laplace’s Law Hypothesis for Gestation
A. Malfunction of the Lower Uterine Pole (LUP)
Transformation of the cervix to the LUS by an active mechanism not only imposes chronic stress on the cervical collagen but also affects all other LUP components. The chronic stress on the LUS may cause its malfunction. Although the likelihood of preterm delivery increases with an increased frequency of uterine contractions, measurement of this frequency is not clinically useful for predicting preterm birth The risk of spontaneous preterm delivery is increased in women who are found to have a short cervix using vaginal ultrasonography during pregnancy [27]. Fetal fibronectin is an excellent test for predicting spontaneous preterm birth [124]. Short cervical length (CL) with an absent cervical gland area (CGA) represents an independent predictor of preterm delivery (PTD), similar to fetal fibronectin (fFN) [125]. Increased frequency of uterine contractions, short cervical length, fibronectin in cervicovaginal secretions, and absence of cervical gland area were four independent components, tests, or factors associated with the same obstetric condition: preterm labor. The statistical significance was comparable.
The link among these evidence-based studies of the LUP components is the malfunction of the cervix secondary to its transformation into the LUS due to uterine contractions that cause preterm birth.
B. Rupture of Fetal Membranes (FMs)
According to our hypothesis, complete loss of cervical resistance is the primary mechanism that initiates labor. In most pregnancies, labor begins at term in the presence of intact FMs, in full-term pregnancy [126]. The rupture of FMs is discussed in the following clinical situations:
a) Spontaneous rupture of FMs at full term. In our hypothesis, the rupture of FMs is a sign of pregnancy termination due to EUWT failure, and the time between the rupture of FMs and labor [122] is the transition phase of pregnancy according to our hypothesis.
b) Preterm premature rupture of membranes accounts for 25%–40% of preterm births [29,127]. Most women with preterm premature rupture of membranes begin labor spontaneously within several days. However, a small proportion of women do not deliver for weeks or months [128].
c) Amniotomy of shortening spontaneous labor: Based on the findings of this review, we do not recommend that amniotomy should be introduced routinely as part of standard labor management and care [129].
d) Amniotomy of induction of labor (IOL): There is insufficient evidence regarding the effects of amniotomy alone (deliberate rupture of membranes) to induce labor [130].
The above evidence showed that amniotomy is not clinically effective either as a method for IOL or augmentation in spontaneous labor, which could be explained according to UMTHG:
• As an IOL method, amniotomy has no direct effect on the myometrium, as it indicates only the transition phase, which ranges from a few hours to a few days.
• As augmentation in spontaneous labor, amniotomy does not directly affect myometrial contraction in the active contracting myometrium.
C. Fate of Human Conception
Gravett et al. [131] have repeatedly stated what Romero et al. have stated succinctly [132]: “few biological processes as central to the survival of a species as parturition are so incompletely understood.” Two compelling principles emerge from the current understanding of pregnancy and parturition. First, labour represents a natural continuum of processes that begin at implantation and culminate with the return of the uterus, after involution, to its non-pregnant state [93,133]. The second compelling principle is that stillbirth and preterm birth are outcomes of multifactorial etiologies. Stillbirth and preterm birth represent the common outcomes of various causes, each associated with distinct biological pathways [134]. Globally, there are approximately 180 million pregnancies annually, and most of these are evacuated in due course, and the uterus rarely fails to evacuate itself. The link between all these facts is the EUWT and AIMCC interactions which represent the pathophysiology of pregnancy. It also ensures that the maintenance of pregnancy is autonomic and intrinsic, and the termination of pregnancy is autonomic, intrinsic, obligatory, and deterministic, followed by involution.
Clinical Implications
A. Stretch-Dependent Inhibitory System Malfunction
The obstetric literature is replete with overwhelming evidence that multiple gestations, IUGR, intrauterine fetal death, decreased P/E ratio, increased estrogen cascade, loss of cervical strength, rupture of FMs, and increased or decreased amniotic fluid initiate parturition; however, the underlying mechanism is unknown. All these conditions contribute to and modulate EUWT. The ultimate outcomes of all these conditions are EUWT and SDIUS failure and termination of pregnancy.
B. Current Treatment of Pre-term Labor
Cervical cerclage benefits a select group of patients Systematic reviews and meta-analyses have reaffirmed that vaginal progesterone reduces the risk of preterm birth in singleton pregnancy with a short cervix [135], and this hypothesis showed that progesterone decreases uterine wall plasticity, which increases IUP and EUWT [38] and may induce uterine relaxation through a stretch-dependent inhibitory system that delays or prevents premature loss of cervical strength. A recent randomized controlled trial of asymptomatic singleton pregnancies with a short cervix (≤25 mm) observed at the 18–22-week scan found that the use of the Arabin cervical pessary reduces the occurrence of spontaneous delivery before 34 weeks by approximately four-fold [136] and an MRI-based study has confirmed that this is due to a mechanical effect on the uterocervical angle According to UMTHG, the mechanical effect of the Arabian cervical pessary at the uterocervical angle may cause stimulatory system malfunction and prevent early loss of cervical strength secondary to its transformation into the LUS. The link among all these therapeutic preterm strategies is the focus on preventing or delaying premature EUWT failure through different therapeutic modalities for different UWT components.
C. Stimulatory System Malfunction
Inaccurate dating is the most common cause of prolonged pregnancy [19]. When a post-term pregnancy truly exists, its cause is mostly unknown. According to our hypothesis, the timing of birth is due to EUWT failure secondary to a complete loss of cervical strength. If the cervix is short, it increases the risk of spontaneous preterm delivery [27]; if the cervix is long mid-pregnancy, it increases the risk of a caesarean section at term [138]. Long cervix at term is associated with the post-term, and in the 41–50 mm cervical length group, 0% of patients delivered at 40 weeks, while in the group with a cervical length of 1–10 mm, 100% of the patients delivered within 2 weeks [68]. So, the link between cervical length and initiation of labor is strong. In this hypothesis, the pathophysiology of post-term pregnancy may be due to the failure of the transformation of the cervix into the LUS by the stimulatory system in the third trimester which makes the cervix lose its strength, resulting in EUWT failure, that initiates labor.
From my clinical experience, I noticed that lightening (when the fetus settles or drops lower) is associated with normal delivery at full term or easy instrumental deliveries. This study was started in 2000 to answer two clinical questions:
1. Why is lightening associated with normal delivery at full term or easy instrumental delivery?
Or
2. What is the mysterious uterine interaction in the third trimester that causes lightening and initiates full-term labor?
This hypothesis suggests that the successful transformation of the cervix, not the isthmus, into the LUS may be the answer to these two questions.
Parturition
MTCI and Labor
Typical uterine contractions consist of a slow rise and fall in tension lasting approximately 1 min [139]. Beyond the inherent myogenic properties of muscles, neurogenic and hormonal control systems are superimposed to initiate, augment, and suppress myometrial activity [140,141]. The myometrium is a phasic smooth muscle that exhibits spontaneous and agonist-induced contractions [142]. The rhythmicity and generation of such contractions are closely related to the generation of slow waves and superimposed action potentials [143,144]. Progressive myometrial shortening is an obligatory part of both normal and premature labor. Over the 12-hour course of labor, the decrease in myometrial length was equivalent to an average decrease of 1% every 10 min [47].
Oxytocin (OT) can directly induce myometrial contractions through PLC, which activates calcium channels and releases calcium from intracellular stores [145,146]. However, OT receptor antagonists do not prevent the normal timing of the onset of labor. Successful pregnancies were achieved in mice by mating OT-deficient females with OT-deficient males [147,148]. Type 2 MEL receptors (MT2R) and OTR are G protein-coupled receptors that couple with the G-subunit q/11 [149,150]. Similar upregulation of myometrial receptors for MEL and oxytocin occurs at labor onset [151]. There are striking similarities between the MEL regulation of OTR mRNA expression and the regulation of OTR mRNA expression by oxytocin [152,153], leading us to explore further the similarities between the MEL and oxytocin signaling pathways in the myometrium. In addition to myometrial agonist-induced melatonin [109], oxytocin secretion [110] is increased nocturnally.
During labor, the expression of both prostaglandin contractants and relaxants increases. PGF2α, secreted during labor in all species studied to date, including marsupials, acts as a universal myometrial contractant [8]. The expression of PGF2α is significantly correlated with the progression of labor; however, there was no significant difference between the expression in live and dead fetuses [154]. Early studies have shown that the concentration of PGI2 (6-keto-PGF1a) increases significantly in the amniotic fluid [155], maternal plasma [156], and myometrium [157,158], during labor, presenting a paradox because its synthesis increases prior to and during labor [156,159]. After the onset of labor, the plasma PGI2 concentration increases further, accompanied by an increase in PGE2 and PGF2α concentrations [156,160], although the augmentation of PGI2 secretion is not as prominent as that of PGE2 and PGF2α [160]. However, the physiological role of myometrial PGI2 during labor is controversial. For example, PGI2 may prevent the overstimulation of myometrial cells during labor [161]. The synchronous activity of myometrial cells results in powerful contractions that are needed to expel the fetus. Intervening periods of relaxation are equally important because they allow blood flow to the fetus (the blood flow to the foetus decreases during contraction and increases during relaxation) Figure 9 links the above evidence-based research in a novel and robust way for the autonomic control (AIMCC) of the uterus during labor secondary to MTCI. Regarding uterine function during labor, the uterus should not only contract but must also relax to keep the fetus alive; MTCI seems to be the intrinsic and autonomic mechanism that protects the fetus against hypoxia and causes the progress of labor.
The First Stage of Labor
There is a major difference between the current hypothesis and our hypothesis regarding the following:
• How does the first stage of labor progress?
• How does the cervix dilate during the first stage of labor?
• What is the biological mechanism of the first stage of labor?
The answer to these questions will come through the following topics:
• What is the origin of the LUS?
• What is the mechanism of formation of the LUS?
• When is the LUS formation completed?
• What are the boundaries of the LUS?
The First stage of labor with the current hypothesis:
1. The origin and formation of the LUS.
We have argued at the beginning of this script, the isthmus of the cervix does not exist embryologically [3,4], anatomically [5], histologically [6], or radiologically [7].
According to the current hypothesis (163) the mechanism of formation of the LUS from the isthmus is confusing. (Figure 10) Therefore, the following questions arise:
a) The Ext. os. does not move from its anatomical site as in a non-pregnant uterus, as shown in A figure 10.
b) How do the Ext. os and vaginal wall stick together in the second stage of labor with full formation of the LUS, as shown in B figure 2?
c) The anatomical internal os fixed in its anatomical site as a non-pregnant uterus, but with evidence-based, the cervix shortens and anatomical internal os moves down and the cervical funnelling depth opens directly into the LUS [68,69].
d) Where is the Ext. os–cervicovaginal junction distance in the second stage of labor?
e) How and where does the Ext. os–cervicovaginal junction distance disappear in the second stage and instantly appear after delivery with a full, long, and deformed length?
f) Comparison between the anatomical site of Ext os in the second stage of labor and instantly after foetal delivery: How does the Ext os, which is stuck to the vagina during the second stage, appear clinically down to the vaginal introitus instantly after foetal delivery?
g) How is the LUS derived from the isthmus, and does it extend from the anatomical cervical internal os to the physiologic retraction ring? Where is the histological internal os?
2. How does the cervix dilate during first stage of labor?
It remains unclear. The anatomical relationship between the human cervix, the uterine body, and the LUS is the subject of conflicting theories [164]. Some believe that the cervix is pulled up during human parturition to become part of the LUS [165], whereas others suggest that the lower uterine body extends downward towards the cervix to cover the inferior pole of the foetus [166]. Some argue that dilation occurs through direct traction of the myometrium on the cervix, and others believe the “fundal dominant” uterine contractions “push” the foetus through the cervix (a “piston effect”) [167,168]. Lindgrenm [161-171] observed head-to-cervix pressures four times as high as the intrauterine pressure. However, all researchers observed a similar force/ pressure distribution along a meridian of the foetal head, with maximal force/pressure at the level of the largest diameter (equator), decreasing towards the external os [172]. According to the current hypothesis, the cervix does not seem to dilate due to the piston effect. How then does the cervix dilate during labor? It remains unknown. Additionally, we are questioning the current hypothesis that the LUS is derived from the isthmus.
3. Cervical involution
The cervix projects to the vagina and is surrounded by a gutter-like fornix. It is penetrated by the endocervical canal, which connects the uterine cavity to the vagina. The canal is fusiform in shape and flattened from the back; it measures 7 across at its widest part and is approximately 3 cm long [173]. Remodeling of the cervix allows the cervix to perform the complex task of maintaining a pregnancy to term. A later "destructive cervical process", through which the cervix dilates to facilitate delivery [174]. The diameter of the lower end of the external os reaches 100 mm in the second stage of labor [175]. This means that cervix dilates more than 15 times its original diameter. No other known organs in the human body can dilate to more than 15 times their original diameter fully recover and are guaranteed for life. Therefore, the mechanisms underlying the effects of cervical dilatation, involution, and recovery remain poorly understood.
4. The First stage of labor with Laplace’s law hypothesis
• To understand the progress and cervical dilation during the first stage of labor, we must answer the following questions:
• What is the origin of the LUS?
• What are the components of the uterine contractions?
• What are the stages of the formation of LUS?
• What are the clinical and radiological signs of the mechanism of LUS formation?
• What is the clinical importance of the formation of the LUS?
We previously presented the evidence that supports the transformation of the cervix into the LUS. The transformation of the cervix into the LUS has interactive components, two timing phases, two clinical phases, and two radiological presentations, followed by the reversal of all these changes. The cervical transformation into the LUS clinically appears as cervical ripening, shortening, and effacement [75,176], and radiologically as the YVU pattern formation [68,78] and inverted U pattern formation. (Animation 1 and Figure 4,11).
Stages of the formation of the LUS:
1. The third trimester: the cervix starts transformation into the LUS during the third trimester. (see changes in the lower uterine pole and the cervical changes in the third trimester).
2. The first stage of labor:
A. Cervical effacement: Friedman’s graphical representation represents the rate of normal labor during latent and active labor [177], where in the latent phase the cervix dilates slowly and in the active phase it dilates much faster. Zilianti et al. [78] described the appearance of cervical effacement, as observed by transperineal sonography, as a progression of the letters T, Y, V, and U. According to MTHG, labor initiation is not only due to cervical failure with full cervical effacement and U-pattern formation but it has multiple causes due to the possible malfunction of different components of EUWT. Therefore, some patients will start labor and the cervix in a different stage of the TYV pattern, it should be effaced first to reach the U pattern according to Zilianti et al. [78], and then it will start to dilate while some patients may start labor while the cervix is in U pattern, thus, they will start to dilate straightaway.
B. Cervical dilatation: There is a mathematical relationship between the uterine contraction, the cervical dilatation, and the head descent [86,87]. The uterus, the cervix, and the fetal head are the components of DIDUCI. This hypothesis proposes that after full cervical effacement in the first stage of labor, the external cervical os will be pulled and inverted inside up to complete the transformation of the cervix into the LUS. This will turn the bottom of the U pattern into the midsegment of the LUS, and this biological mechanism will appear radiologically as an inverted U pattern (∩) and clinically as progressive cervical dilation. Complete inverted pattern formation clinically is the full cervical dilatation. Why do we call it an inverted U pattern? The lowest part of the cervix is the external cervical os, and at the same time, it is the bottom of the U pattern. The external cervical os will be pulled up, and the bottom of the U pattern is inverted inside up to the midsegment of the LUS. Late labor means that most of the cervical tissues have transformed into the LUS, which is why the scar of the Cesarean section appears deep in the cervix after reversal and involution. We consider an inverted U pattern as a continuation of the TYVU pattern formation and the radiological completion of the transformation of the cervix into the LUS. (Animation 1).
The mechanism of cervical effacement and dilation through the YVU and inverted U pattern hypothesis might explain why obstetricians prevent women in labor from bearing down during the first stage, as bearing down does not affect cervical dilatation because the cervical tissues are moving upward. This mechanism may also explain why the cervix dilates during the first stage of labor despite the head-to-cervix pressure being four times as high as the intrauterine pressure [169-171] and researchers have observed a similar force/pressure distribution along a meridian of the fetal head, with maximal force/pressure at the level of the largest diameter (equator) and decreasing toward the external os [172].
So, in summary, cervical transformation into the LUS has clinical and radiological presentations and a two-phase timing, starting in the third trimester and completing during the first stage of labor. Cervical shortening, ripening, and effacement during the third trimester appear radiologically as TYVU pattern formation and transformation completion in the first labor stage. It will appear clinically as progressive cervical dilation and radiologically as an inverted U pattern formation.
3. The second stage of labor: It starts after the completion of the formation of the LUS. The boundaries of the LUS, appear as a wedge-shaped birth canal that extends from the vaginal vault (cervicovaginal junction) caudally into the physiological retraction ring cranially, and the external cervical os lies in between them. A caesarean section in late labor is associated with a uterine scar in the endocervical canal [178]. Late labor means that most of the cervical tissues have transformed into the LUS, which is why the scar of the caesarean section (CS) appears deep in the cervix after its reversal and involution. Drawing number 3 or 4 in Figure 11 is labor as late as it gets, and if you cut incision for CS at this stage, you will cut really through the endocervical canal, leaving a scar on the endocervix after it is reversed. (Animation 1).
4. The third stage of labor (Reversal Phase of the Cervix) All these cervical changes reverse instantly after foetal delivery, and the cervix returns to its anatomical site and regains its fully anatomical shape. The best definition that I can give for the lower uterine segment is in the words of Bayer: “a portion of the uterus, which before parturition resembles the body, and after it, the cervix.” [2]. The breakthrough in this hypothesis was in 2008, when I clinically discovered the changes in the anatomical site of the external cervical os during and after labor. Our hypothesis is proposing that the cervix transforms into the LUS through the TYVU pattern and an inverted U pattern (∩) with its subsequent reversal.
(Animation 1 and Figure 4, 11).
In summary, the biological mechanism and the progress in the first stage of labor are the successful completion transformation of the cervix into the LUS formation, which begins in the third trimester. The transformation of the cervix into the LUS begins in the third trimester and is completed by the end of the first stage of labor. It has clinical and radiological presentation. Clinically, it is characterized by cervical effacement and dilatation; while radiologically, there is a YVU pattern and inverted U pattern formation. All these cervical changes are reversed instantly after fetal delivery.
The cervix, a unique valve, is distinct in nature and in the mechanism of its function:
• It is mainly composed of collagen.
• It has viscoelastic characteristics.
• Its primary function is to maintain IUP.
• It contributes to the uterine volume when it transforms into the LUS.
• It is a strong and efficient valve.
• It carries a huge weight for its size.
• It keeps its function to the last fiber.
• It maintains the pregnancy until term.
• Its malfunction may cause pre-term or labor dystocia or postterm pregnancy.
• It changes into a birth canal, which starts in the third trimester, and is completed during the first stage of labor.
• The transformation into the CBC (LUS) facilitates fetal delivery.
• The mechanism of transformation into the LUS occurs through TYVU and inverted U pattern formation.
• The force of the transformation is DIDUCI due to contractions of the stimulatory system.
• It has autonomously controlled opening and closure characteristics.
• It starts its transformation into the LUS during the third trimester.
• It completes its transformation by the end of the first stage of labor.
• The clinical cervical dilatation radiologically is an inverted U pattern formation.
• It then reverses instantly after fetal delivery.
• It opens in many weeks, days, and hours.
• It closes instantly after fetal delivery.
• Then it involutes, regenerates, recovers, with a lifetime guarantee.
In summary, the cervix is a unique valve with no similar organ in the human body. It dilates to more than 15 times its original diameter, then involutes, regenerates, and recovers, with a lifetime guarantee. It has an unknown mysterious mechanism of function during pregnancy, which turns out to be very simple. It is a deeply seated pelvic organ and is associated with complex changes during pregnancy, having unexpected opening and closing mechanisms. It opens in weeks by complex mechanism due to upward traction force and closes instantly by reversal of all these changes. We would argue that these mechanisms and functions did not cross the human mind and are very difficult to comprehend.
Clinical implications of Transformation of the Cervix into the LUS.
A. Termination of Pregnancy
Long cervix at term is associated with the post-term, and in the 41–50 mm cervical length group, 0% of patients delivered at 40 weeks, while in the group with a cervical length of 1–10 mm, 100% of the patients delivered within 2 weeks [68]. There is a strong reverse relationship between cervical length and the initiation of labor at full term. In this hypothesis, the successful transformation of most of the cervix into the LUS which leads to EUWT failure in the third trimester is the mechanism of termination of pregnancy and initiation of labor at full term.
B. Labor Dystocia
Dystocia is associated with poor progress during labor and is a major cause of primary caesarean deliveries [18]. However, the biological mechanisms that lead to poor progress during labor are poorly understood. Active management of labor is associated with a lower rate of caesarean section
- which is possible because of a decrease in caesarean deliveries performed for dystocia. Another opinion is that the active management of labor does not reduce the rate of caesarean section deliveries in nulliparous women. However, it is rather associated with a shorter duration of labor and a lower maternal fever [180]. There is conflicting evidence regarding the outcomes of active management in reducing caesarean sections due to dystocia. In this hypothesis, we have shown that initiation of parturition is due to EUWT failure secondary to a complete loss of cervical strength. We would argue that failure of EUWT, other than cervical failure, such as rupture of FM if associated with a long cervix (stimulatory system malfunction), may cause labor dystocia. We would argue that the stimulatory system that fails to transform the 4 cm cervix for 7 weeks (30–37 weeks of gestation) from a T pattern into a U pattern during pregnancy may fail to transform it during a few hours of labor. So, concomitant rupture of fetal membranes (failure of the stretch-dependent inhibitory system) combined with a long cervix (failure of the stimulation system - failure of transformation of the cervix into the LUS) represents a combined two-system failure, which may cause labor dystocia. In this hypothesis, successful transformation of the cervix is essential for successful labor, and most of the cervical transformation into the LUS happens during the third trimester. So, the treatment of labor dystocia should focus on the causes of the stimulatory system's malfunction before labor begins.
In summary, the successful transformation of the cervix into the LUS during late pregnancy by the stimulatory system is the cornerstone of termination of pregnancy and achieving successful labor. The failure of this mechanism alone causes post-term pregnancy; if combined with the failure of the inhibitory system, it results in labor dystocia.
C. Duration of Labor
Attempts to define the normal values and limits of labor duration have yielded inconsistent results, possibly because labor does not readily lend itself to measurements [181]. Most studies on the duration of labor have not provided evidentiary support for their choice of definition of labor onset [182]. In our hypothesis, pregnancy duration is the duration between conception and EUWT failure secondary to the complete loss of cervical strength due to its transformation into the LUS. Also, new definitions are emerging for termination of pregnancy (EUWT failure), initiation of parturition (transition phase), and parturition, which are completely different physiological processes with different mechanisms. The definition of the onset and duration of labor should be revisited and defined in light of these findings.
Suggested Studies and Research for the Hypothesis
A. The anatomical relationship between the urinary bladder and the cervix
The transformation of the cervix into the LUS and its reversal makes it impossible for the urinary bladder to have a direct anatomical relationship with the anterior surface of the supra-vaginal part of the cervix, as described in anatomical textbooks. A study is needed to test and confirm this finding.
B. Cervical and Uterine Ligaments Tension
The fixation of the uterus during contraction to push the fetus causes an increase in the tension and stress of these ligaments, which can be measured by MRI elastography during labor. The strongest risk factor for pelvic organ prolapse is parity. A Swedish population-based study found that the prevalence of genital prolapse was higher in parous women (44%) than in non-parous women [183]. Development of uterine prolapse later in life may be due to recurrent stress of these ligaments during pregnancy and labor due to DIDUCI.
c. How do you diagnose an inverted U-shaped Pattern Formation?
The basis for this study is the observation of the movements of the cervical tissue during pregnancy and labor about the cervicovaginal junction (CVJ), which is fixed by a strong cervical ligament. During the effacement and dilatation phases, all tissues of the vaginal portion of the cervix are pulled upward completely, and there are no cervical tissues below the cervicovaginal junction. Observing the cervical changes in real-time open MRI during labor might help diagnose an inverted U-pattern hypothesis. The basis for the study is as follows:
• In the first stage, a small volume of cervical tissue will be observed below the fixed CVJ. Furthermore, observations of the movements of the cervical tissue upward in relation to the fixed CVJ due to cervical dilatation can be made.
• In the second stage, no cervical tissues will be observed below the fixed CVJ.
• In the third stage, MRI compares the volume of cervical tissues that have been pulled up during the first stage and the amount of reversed cervical tissues after delivery of the fetus in relation to the fixed CVJ.
• All these changes might be observed in four images when the cervix is 4 cm, 8 cm, fully dilated, and after delivery of the fetus. See animations 1 and Figure 4 and 11.
Duration of Pregnancy and Genetic Control
The duration of pregnancy for different animal species is remarkably constant, suggesting a time-measuring process. Such a process most likely involves determining the number of cell divisions that have occurred since the time of fertilization, despite the lack of experimental evidence for this hypothesis [8]. Evidence suggests that in most viviparous animals, the fetus is in control of the timing of birth [184-187]. Horse–donkey crossbreeding experiments in the 1950s showed that the gestational length is intermediate between that of horses (340 days) and donkeys (365 days) [186,188], suggesting a role of the fetal genotype in the initiation of birth. Possibly, the mechanism underlying the timing of birth is encoded in the fetus and is closely linked to and activated when specific prerequisite developmental events occur in the fetus [8]. Considerable evidence suggests that the fetus controls the timing of labor and thus its birth; however, the exact mechanism remains elusive [10]. UMTHG showed that there is overwhelming evidence to support the contention that gestation is maintained and terminated, through EUWT. EUWT is under complex genetic control to maintain a balance, as low UWT can terminate pregnancy [20], and high UWT can compromise fetal health. Braxton Hicks contractions can increase the baseline IUP (10 mm Hg) to 20–30 mm Hg, which is sufficient to significantly affect uteroplacental blood flow [189]; high IUP can compromise fetal growth [20,190,191]. Among natural conceptions in which the date of conception (ovulation) is known, the variation in pregnancy length spans 37 days, even after excluding women with complications or preterm births [192]. We would argue that this wide variation in the duration of pregnancy is due to the complex interaction between four variables: gestational sac, uterus corpus, cervix, and the pelvis, which create, maintain, and terminate EUWT. This hypothesis shows overwhelming evidence to support that EUWT mechano-transduction may be the main mechanism and system that controls uterine function during pregnancy and the hormonal milieu. We would argue that fetal growth is the main biomechanical force behind the creation and maintenance of EUWT, responsible for developing the inhibitory and stimulatory systems that dictate the pregnancy interval and circadian timers. Therefore, the fetus may dictate the timing of birth and pregnancy duration through EUWT. Many laws of physics govern uterine contractility [25]. EUWT is measured by Laplace's Law, which may be the law of physics that governs uterine function during pregnancy. It may explain the cause of the constant pregnancy duration through EUWT, where fetal and gestational sac growth are at its core.
To conclude, this hypothesis shows that the autonomous creation, maintenance, and eventual autonomous termination of EUWT, secondary to light-dark cycle modulation, make the pregnancy interval and the circadian timer constant. At the same time, the malfunction of EUWT changes the birth timing, pregnancy duration, and mode of delivery. EUWT is measured by Laplace's Law and Pascal's principle, which might be the laws of physics that genetically control pregnancy's duration.
Racial and Ethnic Consideration
The outcome of the autonomous gestational cycle of a constant duration is the baby’s average genetic and racial weight. Despite the constant pregnancy duration birth weight showed significant differences between the current reference and other references developed for Chinese or non-Chinese infants [193]. Our observed racial/ethnic differences primarily reflect maternal intrinsic characteristics, such as age and height, possibly shaped by evolutionary processes [194,195]. The creation, maintenance, and termination of EUWT are due to complex multifactorial, genetic, racial, physiological, and environmental factors. Andersen et al. [90] reported a cervical length of 40–44 mm at the 30th week of gestation. Murakawa et al. [66] reported that the measurement was 6 mm shorter on average in Japanese women. Sekiya et al. found that the difference was 3–4 mm, which implies that racial differences exist [70]. Racial/ethnic differences in the baby’s weight may match the genetic and racial differences in the components of EUWT, for example, cervical length.
Human Birth Evolution
Comparative genomic analyses have revealed that approximately 95% of human and chimpanzee DNA sequences are shared [196,197]. However, one of the greatest differences between the two species is in genes related to reproduction. Birth in modern humans is a process unlike that observed in other living species. This is because of interactions between several features of our evolutionary history, such as bipedal locomotion and posture, encephalization, and secondary altricialit [116]. In addition, striking changes in the female pelvis based on adoption of an upright posture by the human ancestor Australopithecus and an increase in the size of the cranium based on the evolution of modern humans have had consequences on the process of parturition [198]. Most, if not all, studies on the evolution of modern human childbirth have focused on the fetal head in relation to the bony pelvis and the upright position. It is unclear whether the function of the cervix, AIMCC [10,47,48], EUWT [20], and evolutionary uterus [81] are related to human evolution and the upright position. The evolution of modern human pregnancy and childbirth should be revisited after the emergence of this hypothesis, especially for EUWT, AIMCC, and the evolution of the uterus, primary cervical function, and DIDUCI.
Summary
Physiologically, human reproduction is divided into two phases; the first phase starts with oogenesis and spermatogenesis and ends with the fertilization of the ovum. The second phase begins when the fertilized ovum is implanted into the uterine cavity and is completed when the baby is born with uterine involution. The last century saw a significant improvement in our understanding of the physiology of the first phase of human reproduction. In 1978, Prof. Robert G. Edwards succeeded in achieving extra-corporeal fertilization through IVF, resulting in the birth of the world's first "test-tube baby." He was awarded the Nobel Prize in Physiology of Medicine in 2010 for his achievement. Implantation of the zygote in the uterine cavity created two major challenges, namely systemic immunological and local challenges. The systemic immunological challenge is to protect the gestational sac and fetus from rejection by the host, i.e., the mother. In 1953, Medawar [199] was the first to propose the concept of the fetal allograft. In 1960, he was awarded the Nobel Prize in Physiology of Medicine for this work. The local biomechanical uterine challenges happen after successful placental implantation without rejection. Consider the changes that it has to undergo to permit the fertilized embryo to implant and penetrate the uterine blood supply, to then undergo extreme hyperplasia and hypertrophy to accommodate the growing fetus while not responding to stretch with contraction, before finally producing an ever more insistent series of contractions to produce 10 cm of dilatation of the cervix and the delivery of the baby and placenta [175]. Local uterine challenges are not fully understood, and the timing of birth and the duration of gestation has not been established yet or systematically studied. Would Laplace's law and EUWT hypothesis be the breakthrough and the answer for the local uterine challenges, the timing of birth, and the pregnancy duration? Laplace's law hypothesis showed that the autonomous creation, maintenance, and eventual termination of EUWT, secondary to light-dark cycle modulation, make gestation an autonomic cycle with constant intervals and circadian timers. At the same time, the malfunction of EUWT changes the birth timing, pregnancy duration, and mode of delivery. Laplace's Law hypothesis for gestation presents a novel perspective of pregnancy and human parturition that has never been suggested before. The current physiology and gestational pathophysiology of gestation should be revised in light of this hypothesis.
Acknowledgment
For the soul of my son, Dr. Ahmad A. Hegazy, who passed away September 1, 2018 (National University of Ireland Galway). Ahmad had been involved in this study since he was a medical student. This project required a vast amount of dedication, research, and hard work; nonetheless, the author acknowledges with gratitude the following advisors and contributors: Dr. Mary Christine De Tavernier, Dr. Mohamad Eltom, Dr. Saada Jaber, Dr. Karim Botros, Dr. Madeleine Robinson, Dr. Azza Alkayyali, and Dr. Aoife McEvoy for their sincere and outstanding efforts to review and re-read my manuscript and their advice on the language and organization of the final manuscript. I also thank Wiley’s editing service for editing the final manuscript and Mrs. Lorraine Manson from the library of Portiuncula University Hospital.
Declarations
Ethics approval and consent to participate: N/A
Availability of data and materials: N/A
Competing interests: The author has no competing interest exists.
Funding: The author received no specific funding for this work.
Authors' Contributions: I am the sole author. I proposed the hypothesis. I designed the study. I conducted a comprehensive literature review related to this hypothesis. I analyzed the published patients’ data related to the hypothesis. I prepared the manuscript draft. I thoroughly reviewed the manuscript. I read and approved the final version of this manuscript.
Tweetable Statement: Laplace's Law and Pascal's Principle dictate the timing of birth and mode of delivery through exponential uterine wall tension and hormonal milieu, as well as their light-dark cycle modulation.
References
- Bisits AM, Smith R, Mesiano S, et al. Inflammatory aetiology of human myometrial activation tested using directed graphs. PLoS Comput Biol 1 (2005): 132-6.
- Smyly W. The Ingleby lecture on the lower uterine segment and the contraction ring. delivered before the medical faculty of the University of Birmingham.: Br Med J 1 (1901): 1189-92.
- Larsen W. Development of the urogenital system. In: Human Embryology. New York, NY: Churchill Livingstone (1993): pp: 235-79.
- Buhimschi CS, Buhimschi IA, Zhao G, et al. Biomechanical properties of the lower uterine segment above and below the reflection of the urinary bladder flap. Obstet Gynecol 109 (2007): 691-700.
- Calder The cervix during pregnancy. In: Chard TGJ, eds. The Uterus. London: Cambridge: Cambidge University Press (1994): pp: 288-305.
- Ferenczy ABW. Anatomy and histology of the cervix. In: Blaustein AU, Kurman RJ eds, Blaustein’s Pathology of the Female Genital Tract New York: Springer Verlag (1987): pp: 141-57.
- Weiss S, Jaermann T, Schmid P, et al. Three-dimensional fiber architecture of the nonpregnant human uterus determined ex vivo using magnetic resonance diffusion tensor imaging. Anat Rec A Discov Mol Cell Evol Biol 288 (2006): 84-90.
- Jenkin G, Young IR. Mechanisms responsible for parturition; the use of experimental models. Anim Reprod Sci 82-83 (2004): 567-81.
- López Bernal A, Rivera J, Europe-Finner GN, et al. Parturition: activation of stimulatory pathways or loss of uterine quiescence? Adv Exp Med Biol 395 (1995): 435-51.
- Norwitz ER, Robinson JN, Challis JR. The control of labor. N Engl J Med 341 (1999): 660-6.
- Garrioch DB. The effect of indomethacin on spontaneous activity in the isolated human myometrium and on the response to oxytocin and prostaglandin. Br J Obstet Gynaecol 85 (1978): 47-52.
- López Bernal A. Mechanisms of labour--biochemical aspects. BJOG 20 (2003): 39-45.
- Cornwell TL, Li J, Sellak H, et al. Reorganization of myofilament proteins and decreased cGMP-dependent protein kinase in the human uterus during pregnancy. J Clin Endocrinol Metab 86 (2001): 3981-8.
- Lye SJ, RJ Paracrine and endocrine control of myometrial activity. In: Gluckman PD, Johnston BM, Nathanielz PW (eds), Advances in Fetal Physiology: Reviews in Honour of GC Liggins Advances in Perinatal Medicine (VII). Ithaca, NY: Perinatology Press (1989):pp: 361-75.
- Bernal AL. Overview of current research in parturition. Exp Physiol 86 (2001): 213-22.
- Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88 (2010): 31-8.
- Garfield RE, Saade G, Buhimschi C, et al. Control and assessment of the uterus and cervix during pregnancy and labour. Hum Reprod Update 4 (1998): 673-95.
- Macara LM, Murphy KW. The contribution of dystocia to the cesarean section rate. Am J Obstet Gynecol 171 (1994): 71-7.
- Crowley P. Interventions for preventing or improving the outcome of delivery at or beyond term. Cochrane Database Syst Rev (2000): CD000170.
- Sokolowski P, Saison F, Giles W, et al. Human uterine wall tension trajectories and the onset of parturition. PLoS One 5 (2010): e11037.
- PaluchEK,NelsonCM,BiaisN,etal.Mechanotransduction: use the force(s). BMC Biol 13 (2015): 47.
- Taggart MJ, Morgan KG. Regulation of the uterine contractile apparatus and cytoskeleton. Semin Cell Dev Biol 18 (2007): 296-304.
- Wu X, Morgan KG, Jones CJ, et al. Myometrial mechanoadaptation during pregnancy: implications for smooth muscle plasticity and remodelling. J Cell Mol Med 12 (2008): 1360-73.
- Anderson AB, Turnbull AC, Murray AM. The relationship between amniotic fluid pressure and uterine wall tension in pregnancy. Am J Obstet Gynecol 97 (1967): 992-7.
- AL C. Force of labour. In: Iffy L, Kaminetzky HA (eds) Principles and practice of obstetrics & perinatology. New York: Wiley; (1981) pp: 761-99.
- Buhimschi CS, Buhimschi IA, Malinow AM, et al. The forces of labour. Fetal Matern Med Rev 14 (2003): 273-307.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med 334 (1996): 567-72.
- Leitich H, Brunbauer M, Kaider A, et al. Cervical length and dilatation of the internal cervical os detected by vaginal ultrasonography as markers for preterm delivery: A systematic review. Am J Obstet Gynecol 181 (1999): 1465-72.
- Tsoi E, Fuchs IB, Rane S, et al. Sonographic measurement of cervical length in threatened preterm labor in singleton pregnancies with intact membranes. Ultrasound Obstet Gynecol 25 (2005): 353-6.
- House M, Sanchez CC, Rice WL, et al. Cervical tissue engineering using silk scaffolds and human cervical cells. Tissue Eng Part A 16 (2010): 2101-12.
- Daskalakis G, Papantoniou N, Mesogitis S, et Management of cervical insufficiency and bulging fetal membranes. Obstet Gynecol 107 (2006): 221-6.
- Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol 201 (2009): 375.e1-8.
- Berghella V, Odibo AO, To MS, et al. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol 106 (2005): 181-9.
- Myers KM, Feltovich H, Mazza E, et al. The mechanical role of the cervix in pregnancy. J Biomech 48 (2015): 1511-23.
- Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA 286 (2001): 1340-8.
- Fisk NM, Ronderos-Dumit D, Tannirandorn Y, et al. Normal amniotic pressure throughout gestation. Br J Obstet Gynaecol 99 (1992): 18-22.
- Smith RE, Henzl MR. Role of mucopolysaccharides and lysosomal hydrolases in endometrial regression following withdrawal of estradiol and chlormadinone acetate. I. Epithelium and stroma. Endocrinology 85 (1969): 50-66.
- Ichikawa S, Tamada H. The effect of oestrogen on uterine plasticity in late pregnant rats. J Reprod Fertil 58 (1980): 165-8.
- Hosoda K, Ichikawa S. Changes of intrauterine pressure in progesterone-treated ovariectomized rats during the second half of pregnancy. Nihon Naibunpi Gakkai Zasshi 60 (1984): 846-51.
- Shynlova O, Oldenhof A, Dorogin A, et al. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod 74 (2006): 839-49.
- Shynlova O, Kwong R, Lye SJ. Mechanical stretch regulates hypertrophic phenotype of the myometrium during pregnancy. Reproduction 139 (2010): 247-53.
- Wray S. Uterine contraction and physiological mechanisms of modulation. Am J Physiol 264 (1993): C1-18.
- Pauerstein CJ, Zauder HL. Autonomic innervation, sex steroids and uterine contractility. Obstet Gynecol Surv 25 (1970): Suppl:617-30.
- Wolfs GM, van Leeuwen M. Electromyographic observations on the human uterus during labour. Acta Obstet Gynecol Scand Suppl 90 (1979): 1-61.
- Haase EB, Buchman J, Tietz AE, et al. Pregnancy-induced uterine neuronal degeneration in the rat. Cell Tissue Res 288 (1997): 293-306.
- Latini C, Frontini A, Morroni M, et al. Remodeling of uterine innervation. Cell Tissue Res 334 (2008): 1-6.
- Hurd WW, Gibbs SG, Ventolini G, et al. Shortening increases spontaneous contractility in myometrium from pregnant women at term. Am J Obstet Gynecol 192 (2005): 1295-301.
- Hurd WW, Gibbs SG, Rudinsky KA. Differential regulation of myometrial prostaglandin production by changes in length. Am J Obstet Gynecol 198 (2008): 225. e1-4.
- Omini C, Pasargiklian R, Folco GC, et al. Pharmacological activity of PGI2 and its metabolite 6-oxo-PGF1alpha on human uterus and fallopian tubes. Prostaglandins 15 (1978): 1045-54.
- Wang YP, Walsh SW, Guo JD, et al. Maternal levels of prostacyclin, thromboxane, vitamin E, and lipid peroxides throughout normal pregnancy. Am J Obstet Gynecol 165 (1991): 1690-4.
- Goodman RP, Killam AP, Brash AR, et al. Prostacyclin production during pregnancy: comparison of production during normal pregnancy and pregnancy complicated by hypertension. Am J Obstet Gynecol 142 (1982): 817-22.
- Miyata A, Hara S, Yokoyama C, et al. Molecular cloning and expression of human prostacyclin synthase. Biochem Biophys Res Commun 200 (1994): 1728-34.
- Sooranna SR, Lee Y, Kim LU, et al. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod 10 (2004): 109-13.
- Kawano M, Mori N. The significance of prostacyclin produced by pregnant rat myometrium: the relationship between myometrial prostacyclin producing activity and passive stretch of myometrium by growing conceptus. Prostaglandins 35 (1988): 305-25.
- Lumsden MA, Baird DT. The effect of intrauterine administration of prostacyclin on the contractility of the non-pregnant uterus in vivo. Prostaglandins 31 (1986): 1011-22.
- Buxton IL, Heyman N, Wu YY, et al. A role of stretch-activated potassium currents in the regulation of uterine smooth muscle contraction. Acta Pharmacol Sin 32 (2011): 758-64.
- Kim JY, Wu WH, Jun JH, et al. Effects of corticotropin-releasing hormone on the expression of adenosine triphosphate-sensitive potassium channels (Kir6.1/ SUR2B) in human term pregnant myometrium. Obstet Gynecol Sci 61 (2018): 14-22.
- Yallampalli C, Dong YL, Gangula PR, et al. Role and regulation of nitric oxide in the uterus during pregnancy and parturition. J Soc Gynecol Investig 5 (1998): 58-67.
- Väisänen-Tommiska M, Nuutila M, Ylikorkala O. Cervical nitric oxide release in women postterm. Obstet Gynecol 103 (2004): 657-62.
- Okawa T, Vedernikov YP, Saade GR, et al. Effect of nitric oxide on contractions of uterine and cervical tissues from pregnant rats. Gynecol Endocrinol 18 (2004): 186-93.
- Chen DC, Ku CH, Huang YC, et al. Urinary nitric oxide metabolite changes in spontaneous and induced onset active labor. Acta Obstet Gynecol Scand 83 (2004): 641-6.
- Buhimschi IA, Yallampalli C, Buhimschi CS, et al. Distinct regulation of nitric oxide and cyclic guanosine monophosphate production by steroid hormones in the rat uterus. Mol Hum Reprod 6 (2000): 404-14.
- Buhimschi I, Yallampalli C, Dong YL, et al. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am J Obstet Gynecol 172 (1995): 1577-84.
- Figueroa JP, Mahan S, Poore ER, et al. Characteristics and analysis of uterine electromyographic activity in the pregnant sheep. Am J Obstet Gynecol 151 (1985): 524-31.
- Iams JD, Johnson FF, Sonek J, et al. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol 172 (1995): 1097-103.
- Murakawa H, Utumi T, Hasegawa I, et al. Evaluation of threatened preterm delivery by transvaginal ultrasonographic measurement of cervical length. Obstet Gynecol 82 (1993): 829-32.
- Paterson-Brown S, Fisk NM, Edmonds DK, et al. Preinduction cervical assessment by Bishop's score and transvaginal ultrasound. Eur J Obstet Gynecol Reprod Biol 40 (1991): 17-23.
- Ramanathan G, Yu C, Osei E, et al. Ultrasound examination at 37 weeks' gestation in the prediction of pregnancy outcome: the value of cervical assessment. Ultrasound Obstet Gynecol 22 (2003): 598-603.
- Mancuso MS, Owen J. Prevention of preterm birth based on a short cervix: cerclage. Semin Perinatol 33 (2009): 325-33.
- Sekiya T, Ishihara K, Yoshimatsu K, et al. Detection rate of the cervical gland area during pregnancy by transvaginal sonography in the assessment of cervical maturation. Ultrasound Obstet Gynecol 12 (1998): 328-33.
- House M, Bhadelia RA, Myers K, et al. Magnetic resonance imaging of three-dimensional cervical anatomy in the second and third trimester. Eur J Obstet Gynecol Reprod Biol 144 Suppl 1 (2009): S65-9.
- Moore RM, Mansour JM, Redline RW, et al. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta 27 (2006): 1037-51.
- House M, Kaplan DL, Socrate S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin Perinatol 33 (2009): 300-7.
- Word RA, Li XH, Hnat M, et al. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 25 (2007): 69-79.
- Iams JD. Prediction and early detection of preterm labor. Obstet Gynecol 101 (2003): 402-12.
- House M, Paskaleva A, Myers K, et al. The biomechanics of cervical funneling: The effect of stroma properties, anatomic geometry and pelvic forces on funnel formation. American Journal of Obstetrics & Gynecology 191 (2004): S17.
- Paskaleva A, Socrate S, House M. Biomechanics of cervical funneling: Case of cervical incompetence. In: Proceedings of the Summer Bioengineering Conference Vail, CO: 9; ASME Summer Bioengineering Conference (2005).
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med 14 (1995): 719-24.
- Catalin SB, Irina AB, Andrew MM, et al. The forces of labour. Fetal Matern Med Rev 14 (2003): 273-307
- Iams JD, Newman RB, Thom EA, et al. Frequency of uterine contractions and the risk of spontaneous preterm delivery. N Engl J Med 346 (2002): 250-5.
- Noe M, Kunz G, Herbertz M, et al. The cyclic pattern of the immunocytochemical expression of oestrogen and progesterone receptors in human myometrial and endometrial layers: characterization of the endometrial-subendometrial unit. Hum Reprod 14 (1999): 190-7.
- Wetzstein Der uterusmuskel: morphologie. Arch Gynecol 202 (1965): 1-13.
- Huszar G, Naftolin F. The myometrium and uterine cervix in normal and preterm labor. N Engl J Med 311 (1984): 571-81.
- Aguilar HN, Mitchell BF. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update 16 (2010): 725-44.
- Gee H. The interaction between cervix and corpus uteri in the generation of intra-amniotic pressure in labour. Eur J Obstet Gynecol Reprod Biol 16 (1983): 243-52.
- Sallam HN, Abdel-Dayem A, Sakr RA, et al. Mathematical relationships between uterine contractions, cervical dilatation, descent and rotation in spontaneous vertex deliveries. Int J Gynaecol Obstet 64 (1999): 135-9.
- Barnea O, Luria O, Jaffa A, et al. Relations between fetal head descent and cervical dilatation during individual uterine contractions in the active stage of labor. J Obstet Gynaecol Res 35 (2009): 654-9.
- House M, Socrate S. The cervix as a biomechanical structure. Ultrasound Obstet Gynecol 28 (2006): 745-9.
- House M, Paskaleva A, Myers K, et al. The connection between uterine contractions and cervical dilation: the biomechanics of cervical deformation. Am J Obstet Gynecol 193 (2005): S55.
- Andersen HF, Nugent CE, Wanty SD, et al. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol 163 (1990): 859-67.
- Berghella V, Kuhlman K, Weiner S, et al. Cervical funneling: sonographic criteria predictive of preterm delivery. Ultrasound Obstet Gynecol 10 (1997): 161-6.
- Norwitz ERRJ, Repke JT. Labor and delivery. In: Gabbe SG NJ, Simpson JL, et al., editors, Obstetrics: normal and problem pregnancies 4th edition. ed. New York7 ChurchillLivingstone (2001): pp: 353-400.
- Challis JRG, Matthews SG, Gibb W, et al. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21 (2000): 514-50.
- Cunningham FG LK, Bloom SL, Spong CY, et al. Williams obstetrics Chapter 21, Physiology of Labor,. (24th edition) ed. New York: McGraw-Hill Education (2014): pp: 409-16.
- Viswanathan N, Davis FC. Timing of birth in Syrian hamsters. Biol Reprod 47 (1992): 6-10.
- Miller BH, Olson SL, Turek FW, et al. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 14 (2004): 1367-73.
- Reppert SM, Henshaw D, Schwartz WJ, et al. The circadian-gated timing of birth in rats: disruption by maternal SCN lesions or by removal of the fetal brain. Brain Res 403 (1987): 398-402.
- Olcese J, Lozier S, Paradise C. Melatonin and the circadian timing of human parturition. Reprod Sci 20 (2013): 168-74.
- Prasai MJ, Pernicova I, Grant PJ, et al. An endocrinologist's guide to the clock. J Clin Endocrinol Metab 96 (2011): 913-22.
- Imbesi M, Arslan AD, Yildiz S, et al. The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal 'clock' gene expression in striatal neurons in vitro. J Pineal Res 46 (2009): 87-94.
- Balsalobre A. Clock genes in mammalian peripheral tissues. Cell Tissue Res 309 (2002): 193-9.
- Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol 200 (2009): 3-22.
- Blair EM, Liu Y, de Klerk NH, et al. Optimal fetal growth for the Caucasian singleton and assessment of appropriateness of fetal growth: an analysis of a total population perinatal database. BMC Pediatr 5 (2005): 13.
- Harding R, Poore ER, Bailey A, et al. Electromyographic activity of the nonpregnant and pregnant sheep uterus. Am J Obstet Gynecol 142 (1982): 448-57.
- Mesiano S. Yen and Jaffe's Reproductive Endocrinology. In: J. Strauss RB, editors., editor. Part 1 Edocrinology of Reproduction 6th Edition ed. Philadelphia: Elsevier Saunders (2009): pp: 273.
- Patrick J, Challis J, Natale R, et al. Circadian rhythms in maternal plasma cortisol, estrone, estradiol, and estriol at 34 to 35 weeks' gestation. Am J Obstet Gynecol 135 (1979): 791-8.
- Thorburn GD, Hollingworth SA, Hooper SB. The trigger for parturition in sheep: fetal hypothalamus or placenta? J Dev Physiol 15 (1991): 71-9.
- Zahn V, Hattensperger W. Circadian rhythm of pregnancy contractions. Z Geburtshilfe Perinatol 197 (1993): 1-10.
- Wierrani F, Grin W, Hlawka B, et al. Elevated serum melatonin levels during human late pregnancy and labour. J Obstet Gynaecol 17 (1997): 449-51.
- Hirst JJ, Haluska GJ, Cook MJ, et al. Plasma oxytocin and nocturnal uterine activity: maternal but not fetal concentrations increase progressively during late pregnancy and delivery in rhesus monkeys. Am J Obstet Gynecol 169 (1993): 415-22.
- Bossmar T, Osman N, Zilahi E, et al. Expression of the oxytocin gene, but not the vasopressin gene, in the rat uterus during pregnancy: influence of oestradiol and progesterone. J Endocrinol 193 (2007): 121-6.
- Morgan MA, Silavin SL, Wentworth RA, et al. Different patterns of myometrial activity and 24-h rhythms in myometrial contractility in the gravid baboon during the second half of pregnancy. Biol Reprod 46 (1992): 1158-64.
- Bievenue AM, Jenkins SL, Nathanielsz PW. The effects of photoperiod on the switching of myometrial contractility patterns of pregnant baboons: relationship to surgery and parturition. J Soc Gynecol Investig 9 (2002): 27-31.
- Lindow SW, Jha RR, Thompson JW. 24 hour rhythm to the onset of preterm labour. BJOG 107 (2000): 1145-8.
- Rabindran R, Kanwar S, Lindow SW. 24-hour rhythm to the onset of term and preterm labour in twin pregnancies. BJOG 117 (2010): 1656-7.
- Rokas A, Mesiano S, Tamam O, et al. Developing a theoretical evolutionary framework to solve the mystery of parturition initiation. Elife 9 (2020).
- Liggins GC. Parturition in the sheep and the human. Basic Life Sci 4 (1974): 423-43.
- Gross G, Imamura T, Muglia LJ. Gene knockout mice in the study of parturition. J Soc Gynecol Investig 7 (2000): 88-95.
- Gross GA, Imamura T, Luedke C, et al. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci U S A 95 (1998): 11875-9.
- Sugimoto Y, Yamasaki A, Segi E, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science 277 (1997): 681-3.
- Liggins GC, Fairclough RJ, Grieves SA, et al. Parturition in the sheep. Ciba Found Symp (1977): 5-30.
- Krispin E. Management of premature rupture of membranes at term: the need to correct a recurring mistake in articles, chapters, and recommendations of professional organizations. Am J Obstet Gynecol 217 (2017): 661.e1-.e3.
- Oldenhof AD, Shynlova OP, Liu M, et al. Mitogen-activated protein kinases mediate stretch-induced c-fos mRNA expression in myometrial smooth muscle cells. Am J Physiol Cell Physiol 283 (2002): C1530-9.
- Goldenberg RL, Mercer BM, Meis PJ, et al. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol 87 (1996): 643-8.
- Asakura H, Fukami T, Kurashina R, et al. Significance of cervical gland area in predicting preterm birth for patients with threatened preterm delivery: comparison with cervical length and fetal fibronectin. Gynecol Obstet Invest 68 (2009): 1-8.
- Alger LS, Pupkin MJ. Etiology of preterm premature rupture of the membranes. Clin Obstet Gynecol 29 (1986): 758-70.
- Shubert PJ, Diss E, Iams JD. Etiology of preterm premature rupture of membranes. Obstet Gynecol Clin North Am 19 (1992): 251-63.
- Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 159 (1988): 661-6.
- Smyth RM, Alldred SK, Markham C. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev (2013): CD006167.
- Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database Syst Rev (2000): CD002862.
- Gravett MG, Rubens CE, Nunes TM, et al. Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Pregnancy Childbirth 10 (2010): S2.
- Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab 87 (2002): 2431-4.
- Challis JRG. Mechanism of parturition and preterm labor. Obstet Gynecol Surv 55 (2000): 650-60.
- Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 3 (2006): 17-42.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤ 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol 48 (2016): 308-17.
- Goya M, Pratcorona L, Merced C, et al. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomised controlled trial. Lancet 379 (2012): 1800-6.
- Cannie MM, Dobrescu O, Gucciardo L, Strizek B, Ziane S, Sakkas E, et al. Arabin cervical pessary in women at high risk of preterm birth: a magnetic resonance imaging observational follow-up study. Ultrasound Obstet Gynecol 42 (2013): 426-33.
- Smith GC, Celik E, To M, et al. Cervical length at mid-pregnancy and the risk of primary cesarean delivery. N Engl J Med 358 (2008): 1346-53.
- Young RC, Zhang P. Inhibition of in vitro contractions of human myometrium by mibefradil, a T-type calcium channel blocker: support for a model using excitation-contraction coupling, and autocrine and paracrine signaling mechanisms. J Soc Gynecol Investig 12 (2005): e7-12.
- Challis JRG, SJ L. Parturition. In: Knobil E, Neil J, eds. The physiology of reproduction New York: Raven Press; (1994): pp. 985-1031.
- Garfield RE. Cellular and molecular bases for dystocia. Clin Obstet Gynecol 30 (1987): 3-18.
- Tribe RM. Regulation of human myometrial contractility during pregnancy and labour: are calcium homeostatic pathways important? Exp Physiol 86 (2001): 247-54.
- Kawarabayashi T, Kishikawa T, Sugimori H. Effect of oxytocin on spontaneous electrical and mechanical activities in pregnant human myometrium. Am J Obstet Gynecol 155 (1986): 671-6.
- Inoue Y, Nakao K, Okabe K, et al. Some electrical properties of human pregnant myometrium. Am J Obstet Gynecol 162 (1990): 1090-8.
- Carsten ME, Miller JD. A new look at uterine muscle contraction. Am J Obstet Gynecol 157 (1987): 1303-15.
- Rivera J, López Bernal A, Varney M, et al. Inositol 1,4,5-trisphosphate and oxytocin binding in human myometrium. Endocrinology 127 (1990): 155-62.
- Young WS, Shepard E, Amico J, et al. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J Neuroendocrinol 8 (1996): 847-53.
- Nishimori K, Young LJ, Guo Q, et al. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A 93 (1996): 11699-704.
- Masana MI, Dubocovich ML. Melatonin receptor signaling: finding the path through the dark. Sci STKE 2001 (2001): pe39.
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81 (2001): 629-83.
- Sharkey JT, Puttaramu R, Word RA, et al. Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J Clin Endocrinol Metab 94 (2009): 421-7.
- Phaneuf S, Asbóth G, Carrasco MP, et al. Desensitization of oxytocin receptors in human myometrium. Hum Reprod Update 4 (1998): 625-33.
- Sharkey JT, Cable C, Olcese J. Melatonin sensitizes human myometrial cells to oxytocin in a protein kinase C alpha/extracellular-signal regulated kinase-dependent manner. J Clin Endocrinol Metab 95 (2010): 2902-8.
- Manabe Y, Sagawa N, Mori T. Fetal viability does not affect the onset of stretch-induced labor and the increase in amniotic fluid prostaglandin F2 alpha and plasma prostaglandin F2 alpha metabolite levels. Prostaglandins 44 (1992): 119-28.
- Mitchell MD, Keirse MJ, Brunt JD, et al. Concentrations of the prostacyclin metabolite, 6-keto-prostaglandin F1 alpha, in amniotic fluid during late pregnancy and labour. Br J Obstet Gynaecol 86 (1979): 350-3.
- Ylikorkala O, Mäkäräinen L, Viinikka L. Prostacyclin production increases during human parturition. Br J Obstet Gynaecol 88 (1981): 513-6.
- Quaas L, Zahradnik HP, Breckwoldt M. Age-, cycle-and topographic dependency of human myometrial prostaglandin (6-keto-PGF1 alpha, PGF2 alpha)-synthesis in vitro. Prostaglandins 29 (1985): 739-45.
- Moonen P, Klok G, Keirse MJ. Increase in concentrations of prostaglandin endoperoxide synthase and prostacyclin synthase in human myometrium in late pregnancy. Prostaglandins 28 (1984): 309-21.
- Ylikorkala O, Paatero H, Suhonen L, et al. Vaginal and abdominal delivery increases maternal urinary 6-keto-prostaglandin F1 alpha excretion. Br J Obstet Gynaecol 93 (1986): 950-4.
- Davidson BJ, Murray RD, Challis JR, et al. Estrogen, progesterone, prolactin, prostaglandin E2, prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha, and 6-keto-prostaglandin F1 alpha gradients across the uterus in women in labor and not in labor. Am J Obstet Gynecol 157 (1987): 54-8.
- Korita D, Sagawa N, Itoh H, et al. Cyclic mechanical stretch augments prostacyclin production in cultured human uterine myometrial cells from pregnant women: possible involvement of up-regulation of prostacyclin synthase expression. J Clin Endocrinol Metab 87 (2002): 5209-19.
- Smith R. Parturition. N Engl J Med 356 (2007): 271-83.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics (24th edition). Chapter 23, Abnormal labor. New York: McGraw-Hill Education (2014): pp: 456, Figure 23-1.
- JB DL. Changes due to pregnancy. In: The Principles and Practice of Obstetrics 4th ed. Philadelphia: PA: W.B. Saunders; (1925): pp: 178-219.
- Reynolds SR, Hellman LM, Bruns P. Patterns of uterine contractility in women during pregnancy. Obstet Gynecol Surv 3 (1948): 629-46.
- Allman AC, Genevier ES, Johnson MR, et al. Head-to-cervix force: an important physiological variable in labour. 2. Peak active force, peak active pressure and mode of delivery. Br J Obstet Gynaecol 103 (1996): 769-75.
- Allman AC, Genevier ES, Johnson MR, et al. Head-to-cervix force: an important physiological variable in labour. 1. The temporal relation between head-to-cervix force and intrauterine pressure during labour. Br J Obstet Gynaecol 103 (1996): 763-8.
- Caldeyro-Barcia R, Alvarez H, Poseiro J. Normal and abnormal uterine contractility during labor. Triangle 2 (1955): 41-52.
- Lindgren CL, Smyth CN. Measurement and interpretation of the pressures upon the cervix during normal and abnormal labour. J Obstet Gynaecol Br Emp 68 (1961): 901-15.
- Lindgren L. The influence of uterine motility upon cervical dilatation in labor. Am J Obstet Gynecol 117 (1973): 530-6.
- Lindgren L. The concept of pressure in biology and pressure transducers. Acta Obstet Gynecol Scand Suppl 66 (1977): 87-123.
- Antonucci MC, Pitman MC, Eid T, et al. Simultaneous monitoring of head-to-cervix forces, intrauterine pressure and cervical dilatation during labour. Med Eng Phys 19 (1997): 317-26.
- Chamberlain G, Steer P. Basic science in obstetrics: anatomy of the female pelvis. Turnbull's Obstetrics. 3rd ed. London: Churchill Livingstone: Elsevier Health Sciences (2004): 17.
- Ludmir J, Sehdev HM. Anatomy and physiology of the uterine cervix. Clin Obstet Gynecol 43 (2000;): 433-9.
- Wray S. Insights into the uterus. Exp Physiol 92 (2007): 621-31.
- Mitchell BF, Wong S. Changes in 17 beta,20 alpha-hydroxysteroid dehydrogenase activity supporting an increase in the estrogen/progesterone ratio of human fetal membranes at parturition. Am J Obstet Gynecol 168 (1993): 1377-85.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol 6 (1955): 567-89.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of Cesarean section. Ultrasound Obstet Gynecol 57 (2021): 466-70.
- O'Driscoll K, Foley M, MacDonald D. Active management of labor as an alternative to cesarean section for dystocia. Obstet Gynecol 63 (1984): 485-90.
- Frigoletto FD, Lieberman E, Lang JM, et al. A clinical trial of active management of labor. N Engl J Med 333 (1995): 745-50.
- Neal JL, Lowe NK, Ahijevych KL, et al. "Active labor" duration and dilation rates among low-risk, nulliparous women with spontaneous labor onset: a systematic review. J Midwifery Womens Health 55 (2010): 308-18.
- Hanley GE, Munro S, Greyson D, et al. Diagnosing onset of labor: a systematic review of definitions in the research literature. BMC Pregnancy Childbirth 16 (2016): 71.
- Onwude JL. Genital prolapse in women. BMJ Clin Evid (2009).
- Honnebier MB, Nathanielsz PW. Primate parturition and the role of the maternal circadian system. Eur J Obstet Gynecol Reprod Biol 55 (1994): 193-203.
- Challis JRG, WG. Control of parturition. Prenat Neonat Med 1 (1996): 283-91.
- Liggins GC. Initiation of labour. Biol Neonate 55 (1989): 366-75.
- Nathanielsz PW. Comparative studies on the initiation of labor. Eur J Obstet Gynecol Reprod Biol 78 (1998): 127-32.
- Turnbull AC, Anderson AB. Evidence of a foetal role in determining the length of gestation. Postgrad Med J 45 (1969): 65-7.
- Bower S, Campbell S, Vyas S, et al. Braxton-Hicks contractions can alter uteroplacental perfusion. Ultrasound Obstet Gynecol 1 (1991): 46-9.
- Tamada HSI. The effect of estrogen on fetal survival in progesterone- treated ovariectomized rats. Endocrinol Jpn 27 (1980): 163-7.
- Tamada H, Ohtani HJM. The effect of estrogen and progesterone on the volume-pressure curve of the uterine ampulla in late pregnant rats. Endocrinol Jpn 37 (1990): 943-7.
- Jukic AM, Baird DD, Weinberg CR, et al. Length of human pregnancy and contributors to its natural variation. Hum Reprod 28 (2013): 2848-55.
- Dai L, Deng C, Li Y, et al. Birth weight reference percentiles for Chinese. PLoS One 9 (2014): e104779.
- Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol 213 (2015): 449.e1-.e41.
- Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 94 (2014): 1027-76.
- Cheng Z, Ventura M, She X, et al. A genome-wide comparison of recent chimpanzee and human segmental duplications. Nature 437 (2005): 88-93.
- Consortium CSaA. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437 (2005): 69-87.
- Rosenberg KR, Trevathan WR. The evolution of human birth. Sci Am 285 (2001): 72-7.
- Medawar PD. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symposium of the Society for Experimental Biology (1953): 320.

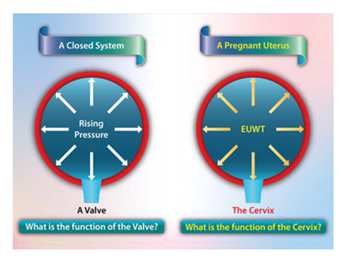
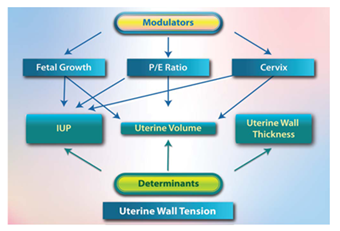
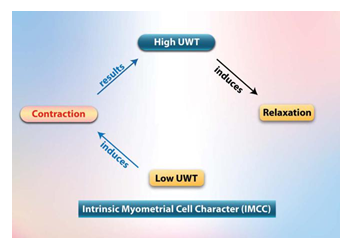
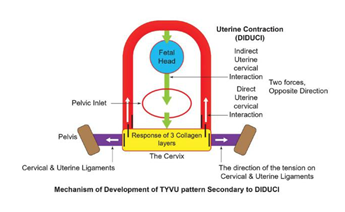
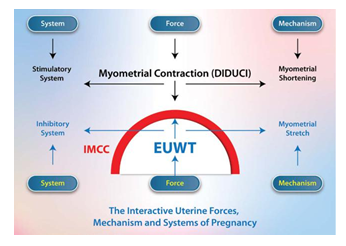
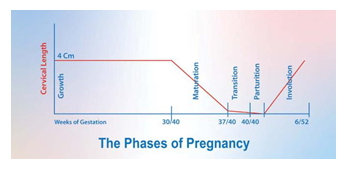
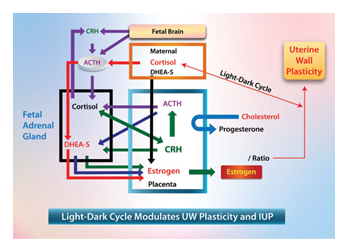
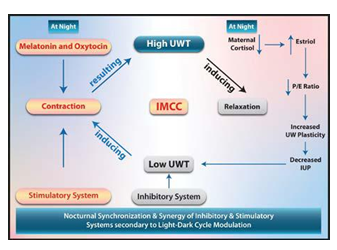
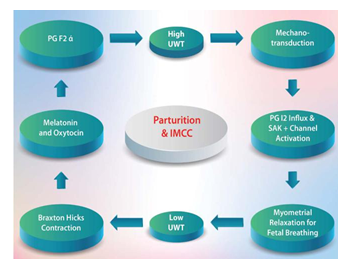
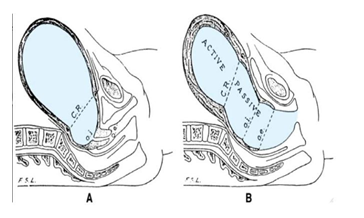
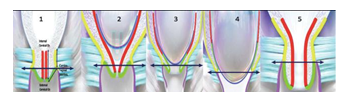

 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
