Monogenic SAMHD1 Aicardi-Goutières Syndrome Type 5; Challenges in Diagnosis and Management: A Case Report
Hamdan Iftikhar Siddiqui*,1, Ali Omar Abdulatef Al Yasari2, Muneeb Mazhar2, Habibullah Abdullah2, Saif Abdullah2, Subhranshu Sekhar Kar3, Rajani Dube4
1General Medicine, Dubai Health Authority, Dubai, UAE
2RAK College of Medical Sciences, RAK Medical & Health Sciences University, UAE
3Professor - Pediatrics, RAK College of Medical Sciences, RAK Medical & Health Sciences University, UAE
4Professor - OB-GYN, RAK College of Medical Sciences, RAK Medical & Health Sciences University, UAE
*Corresponding Author: Hamdan Iftikhar Siddiqui, General Medicine, Dubai Health Authority, Dubai, UAE.
Received: 18 August 2025; Accepted: 22 August 2025; Published: 04 September 2025
Article Information
Citation: Hamdan Iftikhar Siddiqui, Ali Omar Abdulatef Al Yasari, Muneeb Mazhar, Habibullah Abdullah, Saif Abdullah, Subhranshu Sekhar Kar, Rajani Dube. Monogenic SAMHD1 Aicardi- Goutières Syndrome Type 5; Challenges in Diagnosis and Management: A Case Report. Archives of Clinical and Medical Case Reports. 9 (2025): 181-186.
View / Download Pdf Share at FacebookAbstract
Background: Aicardi-Goutières syndrome is a rare autosomal recessive neurogenetic disorder characterized by progressive neurodegeneration, basal ganglia calcifications, leukodystrophy, and chronic cerebrospinal fluid lymphocytosis. Due to the rarity of the condition, there is a lack of specific guidelines for optimal management of these patients. Through this case, we are reporting a mutation in the SAMHD1 gene in the form of homozygous deletion, which is a rare etiology of AGS.
Case Presentation: This report describes an 18-month-old male child with a complex clinical presentation, including microcephaly, developmental delay, quadriplegia, hypotonia, and spasticity. The patient, born to consanguineous parents, was admitted with fever and showed typical neurological features, such as decorticate posturing, a positive Babinski sign, and spastic quadriplegia. Laboratory findings revealed elevated liver enzymes and abnormal lymphocyte distribution. Genetic testing via whole exome sequencing identified a homozygous deletion in exon 2 of the SAMHD1 gene, leading to a pathogenic variant. This mutation is associated with AGS5, contributing to the understanding of AGS genetic variability and emphasizing the importance of early genetic testing for accurate diagnosis. The clinical features align with AGS, supporting the novel detection of SAMHD1 mutations in this context.
Conclusion: This case highlights the critical role of whole genome sequencing in early diagnosis, genetic counseling, and management of AGS. It will contribute to the broader knowledge of the disease spectrum, encouraging further research into AGS.
Keywords
<p>TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1</p>
Article Details
Introduction
Aicardi-Goutières syndrome (AGS) is an autosomal recessive neurodevelopmental disorder, primarily affecting the central nervous system leading to neurological impairment in early childhood [1-3]. The syndrome, first described by Jean Aicardi and Francoise Goutière in 1984, is characterized by intracranial calcifications, particularly in the basal ganglia, along with leukodystrophy, and chronically increased levels of lymphocytes in the cerebrospinal fluid (CSF) [1,4]. AGS is considered a form of congenital cerebral leukodystrophy and is often misdiagnosed due to its clinical overlap with other neurodegenerative disorders, including mimicking congenital infections such as TORCH [5,6]. AGS has been primarily linked to mutations in genes responsible for immune regulation and DNA repair including TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1 [7]. This report describes a novel SAMHD1 mutation in an 18-month-old male. The rationale for reporting a case of AGS lies in the importance of enhancing our understanding of the genetic mutations that underlie the disorder, particularly in diverse populations [8, 9]. This case report aims to highlight a rare mutation in the SAMHD1 gene, which has been identified as a significant contributor to AGS [10,11]. Through a detailed clinical investigation, this case report provides further insight into the phenotypic expression of this condition and underscores the utility of advanced genetic diagnostic tools, such as whole exome sequencing, in identifying rare genetic conditions [7,12]. Additionally, it emphasizes the relevance of consanguinity in the inheritance of AGS, contributing to the broader genetic counseling efforts for affected families [5,6]. By documenting this case, the study seeks to inform future research and improve diagnostic accuracy, which is essential for better patient care and management of AGS [13,14].
Case Presentation
An 18-month-old male child presented to our hospital, for the first time, due to a fever. The patient did not exhibit signs of respiratory distress, dehydration, or meningism. He was born at 38 weeks of gestation via normal vaginal delivery, and there were no intrapartum complications, such as asphyxia, hypoxia, or neonatal complications like jaundice. However, he has a known history of global developmental delay, microcephaly, quadriplegia, and hypotonia. Both parents are healthy, and their marriage was consanguineous. On further questioning, the parents mentioned that the patient had a similarly affected older sister with a history of developmental delay. The child has received vaccinations according to his age, and there were no reports of significant infections or prior hospitalizations. He was then admitted for symptomatic management and observation. Physical examination on admission showed that the patient appeared conscious, without signs of distress or dehydration. However, he was in a decorticate posture. His cardiopulmonary examination was unremarkable, and there was no hepatosplenomegaly. The patient's weight and height were below the normal percentile for his age, indicating growth delay.
On neurological examination, the patient demonstrated:
- • Squinting of the left eye;
- • Positive Babinski sign;
- • Spastic quadriplegia with increased muscle tone;
- • Brisk bilateral deep tendon reflexes;
- • Head circumference of 41 cm, consistent with microcephaly;
- • Positive clonus;
- • Developmental delay in motor milestones, such as sitting, crawling, and standing. The child could not perform fine motor tasks like placing cubes or turning pages.
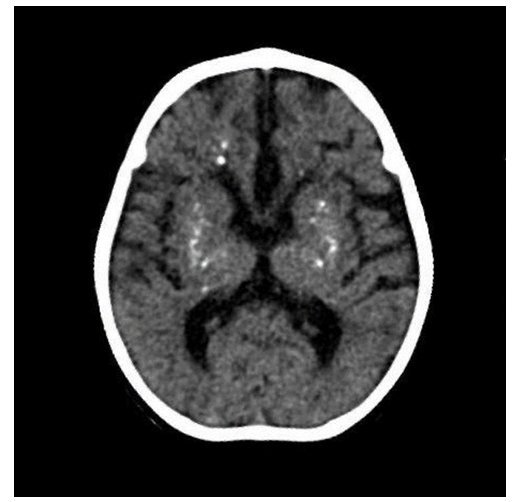
His social and communicative skills were impaired, as he could not track sounds or produce babbling sounds. His hands were clenched, with adducted thumbs, and he was unable to actively grasp or hold objects with both hands. Despite these impairments, he retained the ability to smile, and his cervical correction reflex was positive. The slit lamp and fundus examination were normal, indicating no evidence of ocular abnormalities. Various laboratory tests were undertaken and significant findings are reported in Table 1. Deranged hepatic enzymes (AST, ALT, ALP, GGT) suggested hepatic involvement. Furthermore, a routine full blood count revealed increased lymphocytes and low neutrophils, indicating a shift in the immune cell population, potentially pointing to an inflammatory or immune-mediated process. A TORCH panel (Toxoplasma gondii, Herpes simplex virus 1 and 2, Rubella virus, and Cytomegalovirus) was negative, ruling out common congenital infections. A cerebrospinal fluid (CSF) analysis revealed normal parameters except for increased lymphocytes, indicating CSF lymphocytosis. Additional tests, including electrolyte levels and myocardial zymogram, were normal. Fecal routine and occult blood tests were negative, ruling out gastrointestinal involvement. A thyroid function test (five-item) was also normal, excluding thyroid dysfunction. Deranged laboratory parameters including liver function markers (AST, ALT, ALP, GGT) and blood markers such as lymphocyte and neutrophil percentage (Table 1). A marked increase in liver enzymes, lymphocytosis and neutropenia can be noted. Given the patient's developmental delay, microcephaly, hypotonia, quadriparesis, and a similarly affected older sister, molecular genetic analysis was performed using whole exome sequencing (WES). The sequencing identified a homozygous deletion of exon 2 in the SAMHD1 gene, leading to a frameshift and a premature stop codon. This mutation results in subsequent mRNA degradation or truncation of the SAMHD1 protein. This specific variant had not been previously described in the literature or the general population (gnomAD v2.1.1 controls). It was classified as pathogenic, confirming a diagnosis of Aicardi-Goutières syndrome type 5 (AGS5). Furthermore, a Computed Tomography (CT) scan of the patient’s brain revealed intracranial calcifications, particularly in the basal ganglia [Figure 1], which are typically seen in AGS. AGS5 is characterized by basal ganglia calcifications and leukodystrophy, both of which were suggestive based on the patient's clinical and laboratory findings. The patient's genetic analysis and clinical presentation were consistent with Aicardi-Goutières syndrome, specifically AGS5 caused by pathogenic variants in the SAMHD1 gene (OMIM: 606754). This rare autosomal recessive disorder typically manifests with neurodevelopmental regression, cerebral calcifications, and abnormal immune activation, which the patient exhibited.
Table 1: Laboratory parameters
|
Parameter |
Finding |
Reference Range |
Inference |
|
Aspartate aminotransferase (AST) |
354 U/L |
10-40 U/L |
High |
|
Alanine aminotransferase (ALT) |
399 U/L |
10-40 U/L |
High |
|
Alkaline phosphatase (ALP) |
215 U/L |
90-180 U/L |
High |
|
γ-glutamyl transferase (GGT) |
100 U/L |
5-55 U/L |
High |
|
Lymphocyte percentage |
70.70% |
20-50% |
High |
|
Neutrophil percentage |
20% |
40-60% |
Low |
Treatment
Given the rarity of Aicardi-Goutières syndrome, there is currently no curative treatment [1,7]. The patient's management focused on symptomatic treatment and supportive care. Interventions included:
- • Physical and developmental therapy: Ongoing rehabilitation efforts were initiated to support the patient’s motor development and to manage spasticity;
- • Close monitoring: Regular follow-ups were scheduled to assess liver function, monitor any progression of neurological symptoms, and address any other emerging issues.
Despite early interventions, the prognosis for this patient remains guarded. The patient's developmental delays are expected to persist, and the risk for neurodegeneration over time remains significant [10,14]. The family was counseled on the nature of the disorder, its genetic inheritance, and the implications for other family members, including the patient’s older sister. Genetic counseling was provided to assess the risks for future pregnancies.The identification of the SAMHD1 mutation has allowed for a more precise understanding of the patient's condition, and this case contributes valuable insights into the molecular underpinnings of Aicardi-Goutières syndrome, particularly AGS5.
Outcome
While the patient’s current developmental trajectory is concerning, early genetic diagnosis and targeted interventions are essential in managing this rare and complex syndrome. Continued surveillance and supportive care remain crucial in optimizing the patient’s quality of life. This case report was approved by the hospital and university's ethics committee. Furthermore, written informed consent was obtained from the parents for this publication.
Discussion
The 18-month-old male patient exhibited several clinical features consistent with AGS, including developmental delay, microcephaly, quadriplegia, hypotonia, and a lack of communication milestones (e.g., inability to track sound, no babbling). These signs correlate with the known symptoms of AGS, which commonly include severe developmental impairment, motor dysfunction (e.g., spasticity, dystonia), and cognitive delays. The patient's neurologic examination further highlights abnormal findings, such as a positive Babinski sign, clonus, spastic quadriplegia, and decorticate posture—typical findings in AGS, which frequently present with progressive encephalopathy and neurodegeneration.
A review of the literature confirms that AGS presents with a characteristic set of neurological features, associated laboratory, and genetic analysis including:
- • Spasticity and Hypotonia: As seen in the case, these findings are quite prevalent in AGS, where patients exhibit both peripheral spasticity (increased muscle tone) and truncal hypotonia (reduced muscle tone in the trunk), impairing motor control and postural stability [2,15]. Both features were noted in this case, which is consistent with the expected clinical picture.
- • Seizures: Although not specifically noted in this case, seizures are common in AGS and are often resistant to treatment, varying in frequency and type [4].
- • Developmental Delay: This patient has marked developmental delays, an essential diagnostic feature of AGS. Children with AGS often experience severe delays in motor milestones (e.g., sitting, standing, and walking) and social milestones (e.g., inability to smile, babble, or grasp objects) [2,5].
- • Microcephaly: Microcephaly, often seen in AGS patients, is also confirmed in this case with a head circumference of 41 cm, well below the expected range for this age group. Microcephaly is a critical clinical feature of AGS, often indicative of neurodevelopmental abnormalities [6, 9].
- • Elevated Liver Enzymes: The patient’s liver function tests show significantly elevated levels of glutamic oxaloacetic transaminase (AST), glutamic pyruvic transaminase (ALT), alkaline phosphatase, and γ-glutamyl transferase. Hepatic involvement is well-documented in AGS, and elevated liver enzymes are often present in affected children. However, these markers are non-specific and can be seen in various other systemic disorders;
- • Lymphocytosis: The abnormal lymphocyte percentage (70.7%) in the patient’s emergency blood test along with increased lymphocytes in CSF align with AGS's common finding of chronic CSF lymphocytosis. Elevated lymphocyte levels in CSF are a hallmark of AGS and suggest an ongoing neuroinflammatory process, consistent with the characteristic findings in AGS;
- • Genetic Findings: Genetic testing via WES revealed a homozygous deletion of exon 2 in the SAMHD1 gene, leading to a frameshift mutation and premature stop codon. SAMHD1 mutations are implicated in AGS, specifically in AGS5, which is associated with neuroinflammation, basal ganglia calcifications, and leukodystrophy, as outlined in the literature.7 This discovery is significant because it not only confirms the diagnosis of AGS but also contributes to the growing body of genetic data on AGS, especially regarding the SAMHD1 mutation [9].
Neuroimaging often reveals basal ganglia and intracerebral calcifications, white matter changes, and sometimes cystic degeneration, all of which would be important for confirming the diagnosis [2,5,6]. The chronic CSF lymphocytosis and elevated IFN-alpha levels would further support the diagnosis of AGS, though these specific tests were not mentioned for this patient [1,7,15]. Exploring the genetic basis of diseases helps in studying inheritance patterns, appropriate referrals, genetic counseling, a decision regarding the pregnancy outcomes, preparation, and further plans for the care of the neonate [17-21]. The identification of the SAMHD1 mutation is crucial for both the diagnosis and management of AGS. SAMHD1 is involved in regulating nucleic acid metabolism and immune response, and its deletion leads to defective immune regulation, causing the inflammatory and neurodegenerative features of AGS [10,11]. By identifying the specific mutation, such as in this case, we can:
- Confirm the diagnosis of AGS5 with a genetic basis [10,11].
- Provide genetic counseling to the family, including the implications for future pregnancies and the possibility of AGS in the patient's siblings [3, 8,9]
- Offer a clearer understanding of the prognosis, as patients with SAMHD1 mutations often exhibit poor motor and cognitive development [6, 12, 22].
The management of AGS remains symptomatic and supportive, focusing on optimizing motor function, managing spasticity, and controlling seizures. The case highlights the ongoing challenges of managing a complex neurodegenerative disorder without a clear-cut curative treatment [1,3]. Although not directly discussed in this case, seizures are common and often resistant to treatment. The management approach should include antiepileptic drugs (e.g., levetiracetam, valproate) and possibly ketogenic diet interventions if seizures remain uncontrolled [4,22]. Spasticity could be managed using agents like baclofen, which can improve muscle tone and motor control [2,10]. Physical therapy and occupational therapy are essential in managing developmental delay and motor impairment. Patients often benefit from early intervention and personalized care plans [15, 23].
Conclusions
AGS is of significant concern due to its severe neurological impact and the fact that it is a lifelong condition with no current cure. The disease's rarity and complexity make it difficult to diagnose and manage, often leading to delayed or incorrect diagnoses. Recognizing AGS is crucial because early diagnosis can help provide proper genetic counseling, guide clinical management, and potentially offer insight into novel therapeutic strategies. Furthermore, AGS serves as a valuable model for understanding the broader implications of DNA repair mechanisms and immune regulation in neurodegenerative diseases. This case report highlights a rare mutation in the SAMHD1 gene, which has been identified as a significant contributor to AGS, particularly AGS5. Through a detailed clinical investigation, this case report provides further insight into the phenotypic expression of AGS and underscores the utility of advanced genetic diagnostic tools, such as whole exome sequencing, in identifying rare genetic conditions. Additionally, it emphasizes the relevance of consanguinity in the inheritance of AGS, contributing to the broader genetic counseling efforts for affected families. By documenting this case, the study seeks to inform future research and improve diagnostic accuracy, which is essential for better patient care and management of AGS.
Abbreviations
The following abbreviations are used in this manuscript:
AGS: Aicardi-Goutières Syndrome
CSF: Cerebrospinal Fluid
AGS5: Aicardi-Goutières Syndrome 5
WES: Whole Exome Sequencing
TORCH: Toxoplasmosis, Rubella Cytomegalovirus, Herpes simplex, and HIV
CT: Computed Tomography
SAMHD1: SAM And HD Domain Containing Deoxynucleoside Triphosphate Triphosphohydrolase 1
IFN alpha: Interferon alpha
Reference
- Crow YJ. Aicardi-Goutières Syndrome. In M. P. Adam, J. Feldman, G. M. Mirzaa, et al. (Eds.), GeneReviews® (2005).
- Livingston JH, Stivaros S, van der Knaap MS, et al. Recognizable phenotypes associated with intracranial calcification. Developmental Medicine & Child Neurology 55 (2013): 46–57.
- Crow YJ. Chapter 166 - Aicardi–Goutières syndrome. In O. Dulac, M. Lassonde, & H. B. Sarnat (Eds.), Handbook of Clinical Neurology 113 (2013): 1629–1635.
- Aicardi J, & Goutières F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Annals of Neurology 15 (1984): 49–54.
- Abdel-Salam GMH, Zaki MS, Saleem SN, & et al. Microcephaly, malformation of brain development, and intracranial calcification in sibs: Pseudo-TORCH or a new syndrome. American Journal of Medical Genetics Part A 146A (2008): 2929–2936.
- Livingston JH, Lin JP, Dale RC, et al. A type I interferon signature identifies bilateral striatal necrosis due to mutations in ADAR1. Journal of Medical Genetics 51 (2014): 76–82.
- Rice GI, Forte GM, Szynkiewicz M, et al. Assessment of interferon-related biomarkers in Aicardi-Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: A case-control study. The Lancet Neurology 12 (2013): 1159–1169.
- Sanchis A, Cerveró L, Bataller A, et al. Genetic syndromes mimic congenital infections. The Journal of Pediatrics 146 (2005): 701–705.
- Knoblauch H, Tennstedt C, Brueck W, et al. Two brothers with findings resembling congenital intrauterine infection-like syndrome (pseudo-TORCH syndrome). American Journal of Medical Genetics Part A 120A (2003): 261–265.
- Ramesh V, Bernardi B, Stafa A, et al. Intracerebral large artery disease in Aicardi-Goutières syndrome implicates SAMHD1 in vascular homeostasis. Developmental Medicine and Child Neurology 52 (2010): 725–732.
- Xin B, Jones S, Puffenberger EG, et al. Homozygous mutation in SAMHD1 gene causes cerebral vasculopathy and early onset stroke. Proceedings of the National Academy of Sciences of the United States of America 108 (2011): 5372–5377.
- Thiele H, du Moulin M, Barczyk K, et al. Cerebral arterial stenoses and stroke: Novel features of Aicardi-Goutières syndrome caused by the Arg164X mutation in SAMHD1 are associated with altered cytokine expression. Human Mutation 31 (2010): E1836–E1850.
- Rigby RE, Leitch A, & Jackson AP. Nucleic acid-mediated inflammatory diseases. BioEssays 30 (2008): 833–842.
- Harley ITW, & Sawalha AH. Systemic lupus erythematosus as a genetic disease. Clinical Immunology 236 (2022): 108953.
- Orcesi S, Pessagno A, Biancheri R, et al. Aicardi-Goutières syndrome presenting atypically as a sub-acute leukoencephalopathy. European Journal of Paediatric Neurology 12 (2008): 408–411.
- Kar SS, Dube R, George BT, et al. Role of Genomics in Neonatal Care and Research—A Narrative Review. OBM Genetics 8 (2024).
- Fatima A, Abuhijleh SA, Fatah A, et al. Infantile Neuroaxonal Dystrophy: Case Report and Review of Literature. Medicina 60 (2024): 1322.
- Dube R, Kar SS, Jhancy M, et al. Molecular Basis of Müllerian Agenesis Causing Congenital Uterine Factor Infertility—A Systematic Review. Int J Mol Sci 25 (2024): 120.
- Tayade SD, Mehdi N, Dube R, et al. A rare variant of mullerian agenesis: a case report and review of the literature. J Med Case Reports 18 (2024): 126.
- Sahyouni JK, Odeh LBM, Mulla F, et al. Infantile Sandhoff disease with ventricular septal defect: a case report. J Med Case Reports 16 (2022): 317.
- Ray R, Kar SS, Mahapatro S, et al. A rare cause of seizure-dysgenesis of corpus callosum. Indian Journal of Practical Pediatrics (2009): 411–414.
- Crow YJ, Massey RF, Innes JR, et al. Congenital glaucoma and brain stem atrophy as features of Aicardi-Goutières syndrome. American Journal of Medical Genetics Part A 129A (2004): 303–307.
- Uggetti C, La Piana R, Orcesi S, et al. Aicardi-Goutières syndrome: Neuroradiologic findings and follow-up. AJNR. American Journal of Neuroradiology 30 (2009): 1971–1976.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
