Successful Treatment of Paraneoplastic Dermatomyositis using a Novel IVIg Preparation - A Case Report
Katharina A Kälber, Alexander H Enk, Julia K Winkler*
Department of Dermatology, University Hospital Heidelberg, Heidelberg, Germany
*Corresponding Author: Julia K Winkler, Department of Dermatology, University Hospital Heidelberg, Heidelberg, Germany.
Received: 19 November 2024; Accepted: 25 November 2024; Published: 08 January 2025
Article Information
Citation: Katharina A Kälber, Alexander H Enk, Julia K Winkler. Successful Treatment of Paraneoplastic Dermatomyositis using a Novel IVIg Preparation - A Case Report. Archives of Clinical and Medical Case Reports. 9 (2025): 01-04
View / Download Pdf Share at FacebookAbstract
Dermatomyositis belongs to the group of idiopathic inflammatory myopathies (IIMs). The annual prevalence in adults is 6-7 per 100.000 population. Clinical manifestations include characteristic skin lesions and a progressive muscle weakness. Rapid and sufficient immunosuppressive treatment is demanded. Most patients receive a first-line therapy with oral glucocorticoids. Because of the systemic side effects, steroid-sparing therapy is added. Especially for severe or refractory disease high-dose intravenous immunoglobulins (IVIgs) at a dose of 2 g per kg bodyweight distributed over 2 to 5 days every 4 weeks are a promising and welltolerated treatment option. We report the case of a 75-year-old female patient suffering from severe paraneoplastic dermatomyositis. She achieved significant improvement of skin and muscle symptoms during therapy with a new IVIg preparation, which was very tolerated. Unfortunately, she died four months later since therapy of her lung tumor was refused.
Keywords
Dermatomyositis; Autoimmune disease; High-dose intravenous immunoglobulins; IVIg
Dermatomyositis articles; Autoimmune disease articles; High-dose intravenous immunoglobulins articles; IVIg articles
Article Details
1. Introduction
Dermatomyositis belongs to the idiopathic inflammatory myopathies (IIM), which also include polymyositis, immune-mediated necrotizing myopathy, anti-synthetase syndrome, inclusion body myositis and overlap myositis. The exact pathogenesis and etiology of these acquired diseases remain unknown, but adaptive and innate immune system play a major role. Characteristics include inflammation and cell death of the skeletal muscles. Dermatomyositis is the most common subtype of IIM [1]. Affected patients show a characteristic rash with progressive symmetrical proximal muscle weakness [2]. Dermatological findings include facial swelling with heliotrope erythema and/or plaques appearing over the cheeks. Nailfold capillary changes are found. Gottron’s sign referring to erythema and papules on the extensor finger sides is characteristic as well as V sign, Shawl and Holster signs. In 1887 Wagner first described a patient with dermatomyositis [3]. Dermatomyositis is classified in juvenile dermatomyositis and the more frequent adult dermatomyositis [4]. The incidence of adult dermatomyositis is 0.6-1/100000. Adult dermatomyositis is paraneoplastic in 30 % of cases, which demands an urgent diagnosis [5]. The risk of detecting a tumor is highest with and during the first year of diagnosis and remains elevated for 5 years [6]. Most frequently carcinomas of the gastrointestinal tract and female genital tract, more rarely tumors of the lungs, breasts and nasopharynx are detected and very rarely lymphomas. In paraneoplastic dermatomyositis, symptoms of dermatomyositis may resolve when the tumor is detected. In case of tumor recurrence or metastases worsening of dermatomyositis may occur. All adults with dermatomyositis need to undergo regular tumor screening at least within the first two years after diagnosis. Besides skin and muscle dermatomyositis may involve other organs or tissues. Cardiac involvement in patients with dermatomyositis is an unfavorable prognostic factor [7]. Involvement of smooth muscles occurs in 1/3 of cases, mostly with only mild symptoms. Other organs (lever, kidney) are rarely affected, most common in overlap syndromes with systemic lupus erythematosus or scleroderma. Weakness of oropharyngeal muscles can lead to dysphagia. Lung affection (particularly in antisynthetase syndrome) leads to progressive pulmonary fibrosis, which occurs in up to 30% of cases.
Laboratory findings show elevated creatine kinase (CK, especially MM type) up to 50-fold. Increased aldolase, lactate dehydrogenase (LDH) and liver enzymes may also be found. In the urine creatinine excretion is increased and in severe cases acute renal failure may occur. Antibodies found in dermatomyositis include myositis-specific antibodies (MSAs) and myositis-associated antibodies (MAAs). MSAs are specific to myositis and MAAs can be found in various connective tissue disorders [8]. In 33% of cases antinuclear antibodies (ANAs) are detected. Here, we report on a patient with paraneoplastic dermatomyositis treated with intravenous immunoglobulins (IVIgs).
2. Case Report
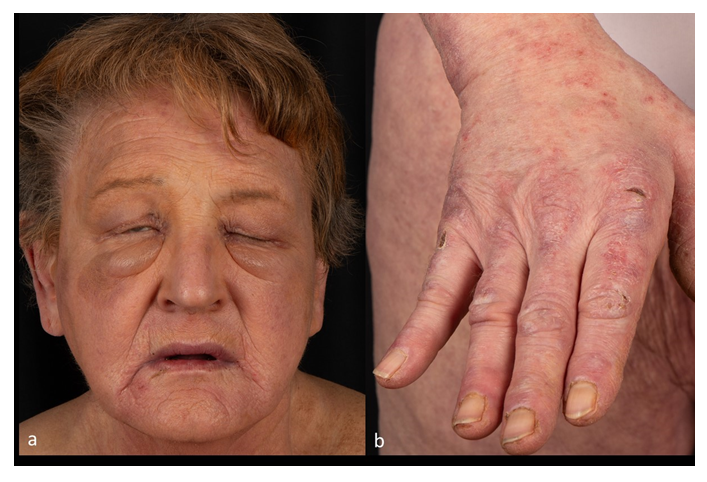
We report on a 75-year-old female patient reporting swollen hands, lips and eyelids in January 2024. She suffered from (joint-)pain in the shoulders and hands. Symptomatic treatment with antihistamines and prednisolone (5 mg daily) did not improve the complaints when she first presented to our dermatology department (Figure 1). Previous illnesses included an arterial hypertension and bronchial asthma, and there was an active nicotine abuse with 20 cigarettes daily. Laboratory findings revealed a significantly increased creatine kinase (>1500 U/l) and the patient was hospitalized with the suspected diagnosis of dermatomyositis. Histopathology from a skin biopsy revealed subepidermal and perivascular lymphocytes, dermal mucin deposition and a prominent basal membrane. Direct immunofluorescence showed subepidermal IgM and C3c positive cytoid bodies, consistent with dermatomyositis. Muscle biopsy showed perifascicular atrophy, upregulation of MHC-I, activated compliment in capillaries and B-cell infiltrates which confirmed the diagnosis of dermatomyositis. Immediately, immunosuppressive therapy with prednisolone 80 mg daily was initiated. Tumor screening with computer tomography revealed a tumor in the left lung and lung cancer was suspected. Gynecological examination was unremarkable and the patient refused further tumor screening with gastro- and colonoscopy. Prednisolone therapy initially improved the patient’s symptoms, which worsened again when prednisolone was reduced to 60 mg daily. Thus, in April 2024 systemic treatment with intravenous immunoglobulins (Yimmugo® 100 g/l Biotest AG, 2 g per kg of body weight distributed over 2 days every four weeks) was started. After the first infusion, the patient developed a superficial venous thrombosis in the area of venous cannula on the right forearm. Anticoagulation with Fondaparinux 2.5 mg subcutaneously for 45 days was initiated and four weeks later thrombophlebitis had completely resolved. After the third cycle of IVIg, the patient presented with a gaping wound on her left knee after having fallen and she developed purulent bursitis. Surgical and antibiotic treatment were demanded. During IVIg therapy her skin and muscle symptoms improved rapidly and dose reduction of prednisolone to 20 mg daily was feasible. Unfortunately, the patient died four months after the start of IVIg therapy (after five cycles), most likely due to the accompanying lung cancer. She had continuously refused any further treatment for her lung tumor.
3. Discussion
Frist-line treatment of dermatomyositis is still based on immunosuppressive therapy with glucocorticoids. Initially high doses of steroids (0.75- 1 mg per kg of body weight) are demanded, in serve conditions intravenous methylprednisolone pulse dose of 1g/day may be required [9]. Glucocorticoid treatment is required for several months to years and adverse events of long-term use include osteoporosis, diabetes and infectious complications [10]. For successful tapering of glucocorticoids adjuvant therapies including azathioprine, mycophenolate mofetil, rituximab or IVIgs may be administered [11].
IVIgs include a pooled preparation of human immunoglobulins obtained from several healthy donors. These are applied for the treatment of antibody deficiencies such as agammaglobulinemia and acquired humoral immune defects as well as autoimmune and inflammatory diseases. IVIg substitution therapy in primary and secondary immunodeficiencies demands lower IVIg doses (400 mg per kg of body weight), for the treatment of autoimmune and inflammatory diseases high doses (1-2 g per kg of body weight) are administered [12]. There are various autoimmune diseases in dermatology, which nowadays may be treated with IVIgs (dermatomyositis, pemphigus vulgaris, pemphigus foliaceus, bullous pemphigoid, systemic vasculitis and systemic lupus erythematosus) [13].
In dermatomyositis IVIgs have acquired market-approval based on results of a prospective, randomized, double-blind, placebo-controlled trial where the percentage of patients with treatment response to IVIg was significantly greater compared with those in the placebo group [14].
Yimmugo® is a novel 10% IVIg preparation with improvements in manufacturing to reduce risk of adverse reactions. Mixing by vibration (“vibromixing”) was introduced to minimize protein damage. Low anticomplementary activity levels were reached due to removal of complement system activator properdin. Due to high levels of functional monomeric antibodies and absence of measurable thrombogenic activity good tolerability and efficacy are achieved [15].
Possible side effects of IVIg include nausea, headaches, tiredness, flushing, malaise and fever. These side effects are easily managed by pre-medication with antihistamines or corticosteroids or reducing the infusion rate. The more severe side effects include renal impairment, aseptic meningitis, hemolytic anemia, arrhythmia and transfusion-related acute lung injury (TRALI) [16]. Especially Yimmugo® was associated with a favorable benefit-risk profile as assessed in patients with chronic immune thrombocytopenia (ITP) [17].
Our case report underlines that IVIg treatment with Yimmugo® is well tolerated in elderly patients. Skin symptoms and muscle weakness in our patient rapidly ameliorated during IVIg therapy. It is of special interest that symptoms improved although the patient denied treatment of her lung tumor. Hence, IVIgs may improve paraneoplastic dermatomyositis even in patients with the underlying malignant disease being untreated. In particular when an additional immunosuppression needs to be avoided, IVIg therapy is a preferred therapy option and should be considered for any patient with paraneoplastic dermatomyositis. Additionally, IVIg therapy is well tolerated, which is even more important in tumor patients already suffering from their malignant disease or from sides effects of oncological therapy. Due to an immediate initiation of a sufficient therapy our patient could live her last months at a good quality of life taking care of herself in her own home.
4. Conclusion
The new 10% IVIg preparation Yimmugo® is also a well-tolerated product, which may improve quality of life in patients with paraneoplastic dermatomyositis.
5. Acknowledgements
Author contribution: All authors had full access to patient data and take responsibility for the integrity and accuracy of the manuscript. All authors contributed to the preparation of the manuscript.
Data Availability: All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics/Ethical Approval: Ethical approval was not required for this case report, in accordance with local and national guidelines.
Conflict of interest: Alexander H. Enk received advisory board honoraria and consultancy fees from Biotest AG. Julia K. Winkler received travel expanse and honoraria from Biotest AG. Katharina A. Kälber has no competing interests.
References
- Mammen AL, Allenbach Y, Stenzel W, et al. 239th ENMC international workshop: classification of dermatomyositis, Amsterdam, the Netherlands, 14–16 December 2018. Neuromuscular Disorders 30 (2020): 70-92.
- Callen JP, Wortmann RL. Dermatomyositis. Clinics in dermatology 24 (2006): 363-73.
- Pascher F. Dermatomyositis. Entzündliche Dermatosen II (1965): 523-49.
- Robinson AB, Reed AM. Clinical features, pathogenesis and treatment of juvenile and adult dermatomyositis. Nature Reviews Rheumatology 7 (2011): 664-75.
- Schlecht N, Sunderkötter C, Niehaus S, et al. Adulte Form der Dermatomyositis-ein Update. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 18 (2020): 995-1014.
- Qiang JK, Kim WB, Baibergenova A, et al. Risk of malignancy in dermatomyositis and polymyositis: a systematic review and meta-analysis. Journal of cutaneous medicine and surgery 21 (2017): 131-6.
- Dankó K, Ponyi A, Constantin T, et al. Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine 83 (2004): 35-42.
- Kilinc OC, Ugurlu S. Clinical features of dermatomyositis patients with anti-TIF1 antibodies: a case based comprehensive review. Autoimmunity Reviews (2023): 103464.
- Anić B, Cerovec M. Polymyositis/dermatomyositis-clinical picture and treatment. Reumatizam 59 (2012): 44-50.
- Patil A, Lu J, Kassir M, et al. Adult and juvenile dermatomyositis treatment. Journal of cosmetic dermatology 22 (2023): 395-401.
- Sasaki H, Kohsaka H. Current diagnosis and treatment of polymyositis and dermatomyositis. Modern rheumatology 28 (2018): 913-21.
- Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. International immunology 29 (2017): 491-8.
- Hoffmann JH, Enk AH. High-dose intravenous immunoglobulin in skin autoimmune disease. Frontiers in Immunology 10 (2019): 1090.
- Aggarwal R, Charles-Schoeman C, Schessl J, et al. Trial of intravenous immune globulin in dermatomyositis. New England Journal of Medicine 387 (2022): 1264-78.
- Duellberg C, Hannappel A, Kistner S, et al. Biochemical characterization of a new 10% IVIG preparation [IgG next generation (BT595)/Yimmugo®] obtained from a manufacturing process preserving IgA/IgM potential of human plasma. Drugs in R&D 23 (2023): 245-55.
- Guo Y, Tian X, Wang X, et al. Adverse effects of immunoglobulin therapy. Frontiers in Immunology 9 (2018): 1299.
- Demeter J, Hamed A, László S, et al. Efficacy and safety of BT595 (10% human intravenous immunoglobulin) in adult patients with chronic immune thrombocytopenia. Transfusion Medicine 33 (2023): 165-73.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
