Therapeutic Drug Monitoring of Antidepressant by Minimally-Invasive Techniques in Eating Disorders Patients: A Clinical Research Report with A Focus on Vortioxetine
Fabio Panariello1*, Tomas Mastellari1, Angela di Gianni1, Francesco Fornasari1, Gianluca Borgiani1, Michele Protti2, Laura Mercolini2, Diana De Ronchi1, Anna Rita Atti1
1Department of Biomedical and Neuromotor Sciences, University of Bologna, Italy
2Research Group of Pharmaco-Toxicological Analysis (PTA Lab), Department of Pharmacy and Biotechnology (FaBiT), University of Bologna, Italy
*Corresponding Author: Fabio Panariello MD, PhD, MSc, Junior Assistant Professor, Department of Biomedical and Neuromotor Sciences, University of Bologna, Italy
Received: 23 April 2021; Accepted: 04 May 2021; Published: 14 May 2021
Article Information
Citation: Fabio Panariello, Tomas Mastellari, Angela di Gianni, Francesco Fornasari, Gianluca Borgiani, Michele Protti, Laura Mercolini, Diana De Ronchi, Anna Rita Atti. Therapeutic Drug Monitoring of Antidepressant by Minimally-Invasive Techniques in Eating Disorders Patients: A Clinical Research Report with A Focus on Vortioxetine. Archives of Clinical and Medical Case Reports 5 (2021): 404-421.
View / Download Pdf Share at FacebookAbstract
Objective: Therapeutic Drug Monitoring (TDM) is an evidence-based practice consistent with the assumption that pharmacological plasmatic concentrations correlate better with clinical effects than prescribed doses of the used drugs. Considering the limited efficacy of Eating Disorders (ED)’s pharmacological treatment and the high rate of adverse effects, TDM may represent a valid tool to check antidepressant drugs (AD) that are frequently prescribed in patients with ED. We aim to test the feasibility of TDM in a real-life setting and to understand the reliability of oral fluid measurements related to whole blood ones.
Methods: We enrolled patients attending the ED outpatient clinic in Bologna with a Body Mass Index (BMI) < 20 or > 30 kg/m2 treated with antidepressants. We collected oral fluid samples and whole blood dried microsamples by finger puncture using VAMS (Volumetric Absorptive Microsampling) technique from patients to determineTDM.
Results: Nineteen patients participated in our study. Participants were treated with Sertraline (N=5), Fluoxetine (N=6), Vortioxetine (N=4), Citalopram (N=2), Escitalopram (N=1), Fluvoxamine (N=1). Preliminary results by our pilot research report show a positive correlation between plasmatic and salivary concentrations only for Vortioxetine. Additionally, only the Vortioxetine shows a pattern of plasmatic concentrations independent from the volume of distribution at the steady-state, even though the wide range of BMI.
Conclusions: Despite the small size of the sample, by considering these preliminary data, we are confident that further studies will allow us to outline that TDM may represent a valid tool to achieve a consistent clinical efficacy of AD in patients diagnosed with ED.
Keywords
<p>Therapeutic Drug Monitoring; Eating Disorders; Antidepressant Drugs</p>
Article Details
1. Introduction
Patients with Eating Disorders (ED) including, among others, Anorexia Nervosa (AN), Bulimia Nervosa (BN) and Binge-Eating Disorder (BED) suffer from a persistent disturbance of eating-related behaviour that results in the altered consumption or absorption of food and that significantly impairs physical health and/or psychosocial functioning [1]. The latest guidelines regarding ED [2] recommend a multidisciplinary intervention, including psychological treatment, dietary advice and medication. Despite the body of research on ED psychopharmacologic treatment has increased lately, there remains a paucity of appropriately sized Randomized Controlled Trials (RCTs) and a great need for further studies on pharmacotherapy drugs in ED [3], and especially in AN [4-6].
Fluoxetine, for example, which is the only Selective Serotonin Reuptake Inhibitor (SSRI) approved to treat BN [7], does not increase patients’ Body Mass Index (BMI) and does not show any significant psychopathological improvement in patients with AN [5]. Current treatment guidelines do not recommend the use of SSRIs as a unique therapeutic approach for AN due to limited efficacy [2, 8] and increased risk of adverse effects [9]; there is a lack of evidence for the use of SSRIs in underweight patients for specific AN-related symptoms [8], although the use of SSRIs may help in relapse prevention and improvement of psychiatric comorbidities (eg. Depression, Anxiety and Obsessive-Compulsive Disorder) [6, 8], above all when weight is restored. Nevertheless, SSRIs are the most common category of psychotropic drugs prescribed in patients with ED [10, 11], even during acute phases, explaining the so-called “research-practice gap” (patients not always receiving treatments rooted in scientific evidence) [12].
It was suggested that SSRIs have decreased efficacy in anorexic patients with low BMI due to starvation-related biochemical changes in the brain [5, 13] and because of inadequate concentration of nutrients, which are necessary for serotonin metabolism14. Nevertheless, nutritional supplements containing tryptophan and essential fatty acids do not seem to increase SSRIs efficacy in underweight patients with AN [15]: the potential reason remains uncertain, but the latest genetic findings suggest that ED may not only be seen as psychiatric but also as metabolic and immune disorders [6, 16]. These changes could also affect the response of ED patients to drugs other than AD. In fact, in a study on patients treated by Risperidone, Paulzen M. and colleagues suggested that extreme low and high BMIs modify psychotropic drugs’ metabolism, in particular pharmacokinetic parameters [17].
Specifically, the authors suggest that patients affected by obesity showed a higher plasma concentration of the active metabolite of Risperidone when compared with underweight patients. Paulzen et al. speculate that this pharmacological behaviour is due to cytochrome P450 activity alterations or differences in P-glycoprotein function. Other studies showed a negative correlation between BMI and plasma concentration of psychotropic drugs and/or their metabolites [18, 19] and these results are explained by the authors as linked to different distribution volumes for lipophilic substances (larger distribution volume in obese subjects leading to inadequate plasma levels and smaller therapeutic efficacy)[19]. Unterecker et al. instead point out the absence of any relationship between body weight and plasma concentrations of antidepressants, highlighting the need for further exploration studies [20].
Considering the altered physical and metabolic condition of patients with a wide range of BMIs, we aimed to investigate the usefulness and feasibility of minimally-invasive and miniaturised bio sampling techniques used for Therapeutic Drug Monitoring (TDM) of psychiatric Central Nervous System (CNS) drugs [21, 22] in outpatients affected by ED. The TDM of SSRIs has already been approved in case of lack of clinical response despite adequate doses, adverse effects using recommended doses, and drugs’ prescription in patients with pharmacokinetically relevant comorbidities (eg. extremely high or low BMI) [23-25].
In this pilot clinical research report, we describe the use of Volumetric Absorptive Microsampling (VAMS) in implementing the TDM in an ED outpatient setting. Our first research question is to verify whether the plasmatic concentration of antidepressant in ED patients with extreme BMI is within normal range or not. Secondly, we applied for the first time in a real-world setting of outpatient eating disorder clinic, the applicability of the VAMS technique for the analysis of antidepressants in whole blood and Oral Fluid (OF) for the purposes of TDM and to describe, in the same setting, the relationship between the blood concentration of AD, the oral posology and the BMIs.
2. Materials and Methods
2.1 Study population
From January 2019 to May 2019, a total of 19 patients consecutively evaluated at the outpatient Unit for Eating Disorders of Bologna University (Northern Italy) were enrolled in the present study. This outpatient unit for ED is an academic clinic specialized in the diagnosis and treatment of ED in adult individuals (≥18 years old). Patients were asked to participate in the study whether they met the following inclusion criteria:
(1) a diagnosis of Eating Disorder according to DSM-5 criteria1,
(2) BMI < 20 kg/m2 or > 30 kg/m2,
(3) age ≥ 18 years
(4) pharmacological treatment (SSRIs or vortioxetine).
Exclusion criteria were the presence of other several mental disorders, in particular schizophrenia, substance abuse and bipolar disorder or significant alterations in renal and/or hepatic function.
2.2 Patient’s assessment
The evaluation protocol at the outpatient unit for ED consisted of three different meetings with a psychiatrist. In the first meeting, socio-demographic and clinical data were collected from the patients and from their medical records; during the second consultation, we filled in a psychopathological assessment including some specific tools for ED; finally, during the third meeting the psychiatrist shared the diagnostic conclusions with the patient and suggested the type of treatment. In case of eligibility for the present study, a fourth meeting was scheduled for the bio sampling session. During this last consultation, additional information was collected by the physician, including the type of antidepressant drug, dose, last intake, first prescription of the treatment and compliance. The information regarding the additional drugs was taken, noted with the dosage and time of intake, and sent to the analysis laboratory, so that any drug interactions potentially altering the results of blood and OF concentrations can be taken into account
2.3 Ethical issue
The present study was approved by the local Ethical Committee (Comitato Etico Indipendente di Area Vasta Emilia Centro, code: CE18135) and all recruited patients signed the informed consent. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975 and its most recent revision.
2.4 Biosampling
Patients participating in the present study were scheduled to be visited at the ED outpatient clinic in the morning between 10 a. m. and 12 a. m. Patients were informed neither to drink nor eat in the 30 minutes before the consultation. The intervention consisted of the microsampling of whole blood in dried form using the VAMS approach and the collection of OF after reaching the steady-state for the antidepressant drug (fourth meeting). In fact, TDM procedures must be carried out under steady-state condition, which is reached within a time of 4-5 half-lives [21]. For different SSRIs and vortioxetine, at least 14 days of treatment are necessary to get to steady-state conditions.
Blood drops were obtained by minimally invasive finger pricking by means of a sterile, disposable needle, while whole blood bio sampling was carried on using specific devices based on VAMS technology. VAMS devices are particularly easy to handle and facilitate the contact between the polymeric head of the device (“tip”) and blood drops. In the present study, three 20-µL tips were obtained for each patient, representing identical sample replicates. Simultaneously, OF was collected by spitting in a dedicated microtube.
VAMS devices were then stored in a tightly closed container and in the dark at room temperature. Storage clamshells provided as part of VAMS packages were used in the present study before the analysis process, which was carried out by the research group of Pharmaco-Toxicological Analysis (Department of Pharmacy and Biotechnology, FaBiT – University of Bologna). On the other hand, OF was stored at the temperature of 0°C before transportation to the Analysis Laboratory. Analysis was carried out by means of fully validated analytical methodologies.
2.5 Materials
Duloxetine hydrochloride (used as the internal standard IS1 for HPLC-UV) and Venlafaxine hydrochloride (used as IS for HPLC-FL), pure powders (all >99% purity); acetonitrile, methanol and dichloromethane (for HPLC, purity: > 99.9%), monobasic potassium phosphate, triethylamine (TEA), phosphoric acid, sodium carbonate and potassium hydroxide (all pure for analysis) were purchased from Sigma Aldrich Italy (Milan, Italy). Clotiapine (used as IS2 for HPLC-UV) pure powders were purchased from LGC Standards (Teddington, Middlesex, UK). Ultrapure water (18. 2 MΩ cm) was obtained by means of a Milli-Q apparatus from Millipore (Milford, MA, USA). ISs stock solutions (1 mg/mL) were prepared by dissolving suitable amounts of pure powders in methanol and kept at -20°C when not in use; the corresponding standard solutions were prepared daily by dilution with the HPLC mobile phase. All solutions were stored protected from light in amber glass vials from Phenomenex (Torrance, CA, USA).
2.6 HPLC-UV- FL instrumentation and conditions
HPLC-UV- FL analysis was performed on a Waters Corporation (Milford, MA, USA) Alliance e2695 chromatographic system with autosampler coupled to a Waters 2998 photodiode array detector and a Jasco FP-2020 spectrofluorometric detector, connected in series. Separations were obtained on a Waters SunFire C18 column (100 x 3.0 mm, 3.5 μm) maintained at room temperature and equipped with a guard column. The mobile phase was a mixture of 33 mM, pH 3.0 aqueous phosphate buffer containing 0.3% TEA (solvent A) and acetonitrile (solvent B), flowing at a constant rate of 1.0 mL/min under gradient conditions. Gradient composition was: 0.0-3.0 min, constant 20% A; 3.1-4.0 min, linear 20%-35% A gradient; 4.1-6.5 min, constant 35% A; 6.6-7.5 min, linear 35%-55% A gradient; 7.6-14.5 min, constant 55% A; 14.6-15.5 linear 55%-20% A gradient, 15.6-17.0 constant 20% A to re-equilibrate the column. Both solvents were filtered on a polyamide filter (47 mm diameter, 0.2 µm) and degassed by ultrasonication. The injection volume was 20 μL. Sertraline (SRT), Norsertraline (NSR) and Vortioxetine (VTX) were detected by UV at 225 nm; Fluoxetine (FLX), Citalopram (CTP), Norfluoxetine (NFL), Dextcitalopram DCT and Diethyldithiocarbamate (DDC) were detected by fluorescence at λem = 235 nm, λexc = 300 nm.
2.7 Blood- and OF-VAMS sampling and pretreatment
For patient sampling, IS spiking was carried out on the VAMS tip by automatic pipetting before the sampling; the tip was then left to dry for 2 h at RT before use. Mitra® VAMS micro samples (20 µL) were provided by Neoteryx (Torrance, CA, USA). A VAMS micro sampler includes a polypropylene handle (about 4 cm long) topped with a small tip (about 2-mm diameter) of a proprietary polymeric porous material.
B-VAMS. The finger prick site was wiped with skin cleansing swabs and dried. Then a disposable sterile lancet was used to prick the fingertip and a blood droplet was allowed to form. Each VAMS tip was held at a 45° angle to the surface of the blood droplet, taking care not to touch the skin. The VAMS devices were held in this position until the whole tips were visible filled with blood (around 5 seconds). The samples were then transferred to the dedicated clamshells, in order to avoid contact with any surface and left to dry at Room Temperature (RT) for 1 hour. VAMS microsamples were thus obtained. Clamshells were stored and transported at RT in sealed polyethene bags containing desiccant until pre-treatment and analysis. For sample pre-treatment, the micro sampler tip was detached from the handle and subjected to ultrasound-assisted extraction (UAE) for 20 min in 1 mL of methanol. The resulting solution was quantitatively transferred into a different vial and brought to dryness in a centrifugal evaporator.
After re-dissolving with 100 µL of HPLC mobile phase, the solution was subjected to Microextraction by packed sorbent (MEPS) pretreatment in an SGE Analytical Science (Melbourne, VIC, Australia) C2 barrel-and-needle (BIN) assembly set up in an SGE eVol XR digital analytical syringe apparatus. The BIN was activated by drawing and discharging 100 µL of methanol 3 times and conditioned with 100 µL of water 3 times. The sample was loaded onto the BIN with 10 draw/discharge cycles at a 5 µL/s speed; the BIN was then washed twice with 100 µL of water and 100 µL of 10 mM, pH 9.0 carbonate buffer/methanol (90/10, V/V) mixture at 20 µL/s. The analyte and the ISs were eluted three times with 200 µL of methanol at 5 µL/s (three cycles). After merging the three eluates, they were brought to dryness, re-dissolved in 100 µL of HPLC mobile phase and analysed by HPLC-UV-FL. OF-VAMS. OF (1 mL) aliquots were centrifuged for 5 min at 6500 x g, then VAMS samplers were used by touching the OF sample surface with the tip and held in position for 10 seconds. Then, the same drying, storage, pre-treatment and analysis workflow as B-VAMS was carried out.
3. Results
1 Sample
Out of 19 patients enrolled, biosample collection and analysis were performed in 6 patients taking Sertraline, 5 patients taking Fluoxetine and 4 patients taking Vortioxetine, 3 patients in treatment with Citalopram and Escitalopram and 1 patient treated with Fluvoxamine. Biological sampling was performed in all patients after reaching steady state. The average time calculated between the start of treatment (Time 0) and the biological sampling is 1.5 months, with a minimum time of 2 weeks for sertraline, vortioxetine and fluoxetine and a maximum time of 24 months for vortioxetine. As shown in table 2, Vortioxetine has been prescribed in our population at a median dosage of 7.5 mg/day, while the median of the sertraline dosage was100 mg/day. Fluoxetine was prescribed at a median dosage of 30 mg/day. The time, expressed in hours, between the last intake of the drug and the collection of the bio-samples was variable (median of 5 hours, minimum value of 30 minutes and maximum of 22 hours). In table 1, additional drugs taken by patients are shown as an indication and simplified. 47% of enrolled patients take other drugs daily.
In table 3 are depicted the findings of our research along with the treatment dose, the duration of the treatment and DeltaTime (DT) between last dose and biosamplig. Starting with Sertraline, we found only a positive correlation between oral dose and blood concentration. Two out of three patients on 100mg/die of Sertraline were matched for BMI value and had a slight difference in DT considering that the peak plasma concentration of Sertraline is after 6-8 hours; their blood concentration was close to 120 ng/ml. The third patients on 100mg/die had a very high BMI and he showed a blood concentration of 111.9 ng/ml probably due to the higher volume of distribution. The last 2 participants on Sertraline, were taking 50mg/die and had a BMI between 17-17.5 (17.2-17.4) but showed a big difference in DT. We found a blood concentration for Sertraline of 59.2 with a DeltaTime of 4,25 hours and of 41.3ng/ml with a Delta Time of 22 hours. Norsertraline showed an opposite trend with a blood concentration of 14.1 ng/ml and 43.2 ng/ml respectively; this is probably due to the pharmacokinetics characteristics of Sertraline. Unfortunately, we didn’t find any correlation between plasma concentration and oral fluid concentration (Figure 1).
Taking in consideration the Fluoxetine, we collected bio-samples from 6 patients with a high variability of oral dose, BMI and DT as you can see from table 1. Probably because of these differences or because of the pharmakocinetics profile of Fluoxetine, we didn’t find any correlation between oral dose, plasma and oral fluid concentration (Figure 1).
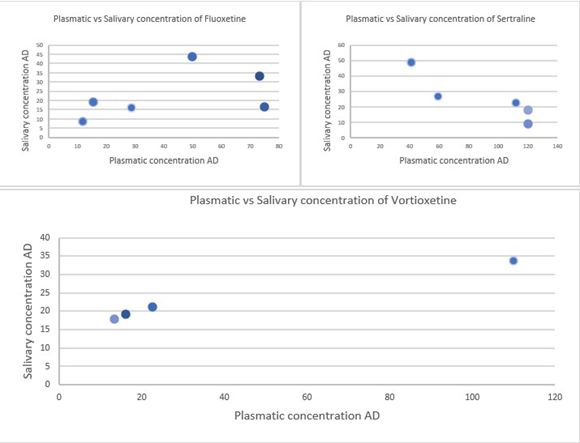
Figure 1: Correlation between plasmatic and salivary concentration of Fluoxetine, Sertraline and Vortioxetine. Plasmatic concentration is reported on the x-axis while salivary concentration on the y-axis.
On the other hand, in terms of correlation between the oral dose, plasma and oral fluid concentration the most informative results were obtained from four patients treated with Vortioxetine. In these patients we outlined a linear correlation between plasma and oral fluid concentration. In addition, we also observed a lack of influence of BMI on plasma concentration. Basically, given the small size of the sample which does not currently allow to attribute to these results a strong significance in terms of statistical significance, for the same oral prescribed dose the same plasma concentrations correspond regardless of BMI. We have focused our attention on Vortioxetine given the pharmacodynamic peculiarities, the specificities of pharmacodynamic behaviour demonstrated in our data collection. We report here the 4 cases of patients taking Vortioxetine following the order we used in table 3. The first patient was a 19 years old woman with a diagnosis of Anorexia Nervosa. She was characterized by a BMI at the time of the collection of 14.1 (height 169 centimetres (cm) and weight 38 kilograms (kg)), no alcohol consumption nor smoking habits. She began Vortioxetine treatment 1 month before reaching a dosage of 3.84 mg per day in the last 2 weeks. The blood and saliva collections were performed 6 hours and half from the last assumption of Vortioxetine and paracetamol along with high-protein food supplements were the only other treatments she took the day before. We found that her whole blood concentration of Vortioxetine was 16.2 ng/ml while that on the saliva was 19.3 ng/ml. The second participant of the study was a 23-year-old woman being diagnosed with Bulimia Nervosa. She showed a BMI of 19.8 (height 154 cm, weight 47 kg) and a smoking habit of 5 cigarettes per day. She started Vortioxetine 2 weeks before sample collection at a posology of 7.68 mg. Bio-sample collection was performed 30 minutes after the last dose of Vortioxetine (the previous one was 24 hours before); delorazepam (0,5 mg) was the one other drug she took the day before. Vortioxetine blood level was 22.6 ng/ml while its saliva level was 21.1 ng/ml.
Thirdly, we enrolled a young woman of 22 years old affected by Anorexia Nervosa having a BMI 19.9 (height 169 cm and weight 59 kilograms). She was a light smoker consuming 5 cigarettes per day while she denied alcohol consumption. Vortioxetine treatment was started 3 months before reaching the dose of 15 mg per day during the last month. She was also taking vitamin supplements of Cobalamin and Folates. We collected bio-samples 1 hour and half after the last dose of Vortioxetine. 49.2 ng/ml was blood concentration and 44 ng/ml was oral fluid concentration.
The last patient who decided to participate in our research, was a 32-year-old male followed in our out-patient clinic for Binge Eating Disorder (BED). He had a BMI 47.3 being 172 cm and weighting 140 kg. He started Vortioxetine treatment 24 months before at a dosage of 3.08 mg per day. He was also on treatment with Allopurinol 150 mg per day to control uremic blood level. We collected blood and saliva 5 hours after the last dose of Vortioxetine. Vortioxetine concentration was 13.3 ng/ml in blood and 17.8 ng/ml in oral fluid.
As you can see in Figure 1, Vortioxetine was the only antidepressant that showed a positive correlation between plasma and saliva concentration. On the contrary Sertraline and Fluoxetine, the most two tested SSRIs, didn’t outline a direct correlation between the concentration in the two biological matrices. Furthermore, as shown in Figure 2, the plasma concentration of Vortioxetine, normalized for a dose of 5 mg/Die, with respect to the BMI, we noticed that in patients with extreme BMI (14.1 kg/m2 and 47.3 kg/m2) the blood concentration was comparable (16.2 ng/dl and 16.62 ng/dl).
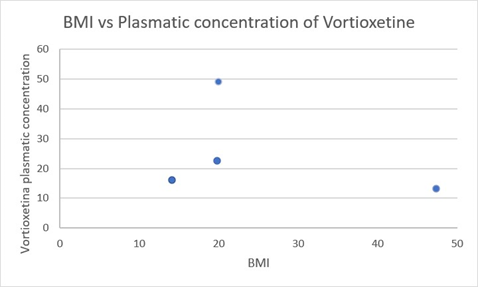
Figure 2: Correlation between BMI and Vortioxetine plasmatic concentration. BMI is reported on the y-axis while Vortioxetine plasmatic concentration on the x-axis.
4. Discussion
The main goal of this study is to highlight a possible relationship between the prescribed doses, the whole blood, and salivary concentration of antidepressants and their active metabolites using minimally invasive TDM methods such as VAMS strategy in a specific population affected by ED. This subset of psychiatric patients is extremely interesting in terms of correlation of peripheral concentration of drugs with oral dosage, because patients with ED have an important variability in terms of BMI and metabolic profile (e.g. protein and lipid concentration, hydration status). It is well known that BMI could affect clinical response to antidepressant drugs [26, 27] and the incidence of side effects affecting the safety of the treatments and the compliance to the therapy. TDM can give us correct information on the concentration of active metabolites of antidepressants in peripheral matrices, net of the effect of liver metabolism and BMI on the pharmacokinetics of psychotropic drugs. It is also important to take into account the frequency of psychiatric and/or medical comorbidities in the cohort of patients affected by ED and this reinforces the rationale of this study, i.e. the use of TDM in this specific population. Moreover, TDM can also lead to reduced healthcare expenses, due to the possibility of better efficacy, increased patient compliance and enhanced safety, leading to a reduction in hospitalizations due to unwanted effects or therapy ineffectiveness [28-31].
VAMS technology is currently a topic of enormous interest for the bioanalytical community and is the subject of numerous studies that have grown exponentially in the last two years [22, 32-35]. VAMS devices were initially marketed for use on whole blood only; this method based on VAMS devices is applied also to the OF for the quantitative analysis of synthetic cathinones [35]. We have carried out an analytical workflow based on VAMS of both whole blood and OF, followed by microextraction by packed sorbent (MEPS) [22] and liquid chromatographic (HPLC) analysis with spectrophotometric (UV) and spectrofluorimetric (FL) detection. We think that our work could help improving the current knowledge of the relationship between the BMI and biological fluid concentrations of antidepressant drugs together with their metabolites in these cohort of patient.
The aim of the present study, evaluating the concentration of antidepressants through VAMS devices both on whole blood and on OF, is to:
1)To examine the relationship between the concentration of drugs (above all Vortioxetine) and any active metabolites in the two biological matrices and oral dosages of the same drugs;
2) To analyse the variability of the concentration of the main antidepressants in use in patients with very varied ED and BMI with a focus on Vortioxetine.
Current findings in the literature concerning the correlation between whole blood and OF concentrations of psychotropic drugs outline information characterized by some limits. Often the data are fragmented, take into account very limited populations, or do not take into account the active metabolites of the drugs: some positive correlations have been highlighted between the whole blood and salivary concentration of monohydroxycarbamazepine [36], carbamazepine, phenytoin and phenobarbital [24, 37]. For amitriptyline, nortriptyline and valproic acid, however, the correlation shown was not significant [24, 38, 39]. For a long time the concentration of drugs in OF has been considered as a reflection of the free component of the drug in the blood, which for many psychotropic drugs is only 10% or less of the total concentration; however, the distribution of drugs between blood and OF largely depends on the pH, which increases when the secretion is stimulated. OF is considered as an interesting and advantageous matrix for TDM studies, in particular due to the non-invasiveness, but data available so far in literature show contrasting results and further studies are necessary to validate the use of OF as a matrix for the TDM of antidepressant drugs [24, 40]. On the other hand, according to the current literature [41], high BMI may also affect treatment response: Khan et al. have found obesity to be associated with a less vigorous treatment response, with an effect more pronounced in males. Moreover, higher BMI was associated with a slower clinical response in the Munich Antidepressant Response Study (MARS) [42].
Several Proof-Of-Concept studies can be found in literature, demonstrating the usefulness of VAMS devices for various compounds and purposes, including the analysis of caffeine, paraxanthine, steroid compounds, 5-methyltetrahydrofolic acid, hydroxyurea, natural and synthetic cannabinoids, cocaine and metabolites, oxycodone and metabolites, asenapine enantiomers and iron isotopes [34, 43-49]. To date, there are no studies on reliability of VAMS technique for the analysis of antidepressant concentrations, neither on blood nor on OF. In this context, this study may be considered as a true innovative pilot study for the usefulness and reliability of VAMS strategy within the TDM of psychotropic drugs, above all in patients with a wide range of BMI and pharmacokinetic characteristics.
Vortioxetine is an antidepressant with multimodal activity currently approved for the treatment of major depressive disorder that is metabolized by cytochrome P450 enzymes and subsequently by uridine diphosphate glucuronosyltransferase. Several evidences from current literature do not show clinically relevant differences in vortioxetine exposure by sex, age, race, body size, and renal or hepatic function. Dose adjustment is only recommended for cytochrome P450 2D6 poor metabolizers and when it is associated with bupropion, a strong cytochrome P450 2D6 inhibitor, and rifampicin, a broad cytochrome P450 inducer. Moreover, pharmacodynamic findings demonstrate that vortioxetine achieves high levels of serotonin transporter occupancy in relevant brain areas and modified abnormal resting state networks in the brain over the therapeutic dose range. Overall, vortioxetine can be administered without major dose adjustments [50].
In this context, our pilot study provides data about application of VAMS for OF analysis, showing a good relationship between whole blood and salivary concentrations for Vortioxetine only even if the sample consists of only 4 patients, with the consequence of having little relevance in terms of statistical significance. The ability to outline the usefulness of VAMS on OF may have been hindered by the relatively small sample size. Future work should seek to enrich the patient sample and to standardise the time of data collecting and the last drug intake.
The second macro-objective of the study concerns the evaluation of the pharmacokinetic behaviour in terms of active metabolite concentration of antidepressant drugs in patients with extreme BMI. The present TDM study of antidepressant drugs in patients with ED and therefore abnormal BMI allows us to:
- Test in clinical practice the use of TDM in the pharmacological treatment of patients with abnormal body weight such as ED patients;
- Contribute to the complex analysis of the factors that modulate the clinical efficacy of psychotropic drugs through a mainly pharmacokinetic perspective in a patient population particularly resistant to drug treatment.
In relation to point 1, we can state that TDM is characterized as a potential Precision Medicine tool, capable of reducing the Trial-and-Error approach and of favoring the therapist's choices in an Evidence-Based prospective above all in outpatient setting where the patients have a low body weight and show ED symptomatology with several psychopathological dimension symptoms. According to the guidelines, and even more so with respect to such fragile patients, often with physical comorbidities and with very low BMI, the psychiatrist’s habit is to prescribe minimal doses of antidepressant drug (SSRIs, vortioxetine, etc. ) and to begin a progressive and slow titration of the dosages. Often, the patient is not affected by any benefit or only reports the appearance of undesirable effects. When the medium-low dose drug treatment is not effective, the therapist must empirically evaluate whether to increase the dosage or to change the type of psychiatric drug. The clinician's resistance in prescribing the highest dosages allowed for a given antidepressant to patients with very reduced BMI recalls the general principles of pharmacology according to which low distribution volumes (AN patients) will correspond to higher blood concentrations for a given dose of drug in ratio to patients with BMI in the normal range, population in which blood concentrations are studied with reference to a given oral dose.
In outpatient setting, a fortuitous trial-and-error process is used to try to reach the optimal dose for the specific patient [25, 51]. This process does not favour the therapeutic alliance and also explains the difficulties of treatment and healing. Instead, the psychiatrist can make targeted decisions relying on non-invasive and timely TDM methods: firstly, to rule out that the patient is not taking the drug at all or that he is taking it incorrectly (as discussed in the introduction part, adherence to pharmacological prescriptions are often scarce in this type of patients particularly ambivalent towards the process of change towards healing); secondly, to increase the dosage when whole blood concentrations of the antidepressant are too low or to change the type of drug if whole blood concentrations are approaching the upper limit of the therapeutic window, without showing the clinical expected results. In patients with high BMI we can also evaluate the whole blood concentration of the drug and therefore modify the daily dose or the molecule according to the pharmacokinetic characteristics of the individual patient.
In consideration of point 2, however, it is necessary to consider the pharmacokinetic variables such as e.g. the absorption, distribution and elimination characteristics of the antidepressant drugs considered in this study, described in the introduction, except for vortioxetine, chosen in this study, for the peculiar pharmacokinetic profile, as well as for the uniqueness of the pharmacodynamic profile [50]. It is crucial also to consider the drug-protein binding, which, in light of the potential alteration of protein concentration in ED patients, may influence plasma free metabolite concentrations.
Overall, the pharmacokinetic factors to be taken into account when describing the concentrations of antidepressants in blood and OF of such patients with particular physical characteristics are numerous and intricate. Ideally, the chemical-physical properties of the drug, its absorption, first pass metabolism, bioavailability, protein binding, distribution in abnormal body volumes (in association with the study of body composition), should be taken into account. The fat content and visceral obesity could explain some differences in the response to antidepressant drugs [19], the passage at the level of the blood-brain barrier to reach the target sites where the action is performed from a pharmacodynamic point of view, as well as the mechanisms of final metabolism and excretion. Added to this is the role of pharmacogenetics and the inter-individual variability, characterized by the different levels of cytochrome activity. Among other things, this activity is modified by obesity and the type of diet, as shown by various studies [52-54].
Our results, in line with recent literature data) [55], support the absence of a significant influence of BMI on drug whole blood concentrations in relation to the oral posology taken. This data partially contrasts with the pharmacokinetic behaviour generally expected by the clinician on the basis of the fundamental principles of pharmacology: in a large distribution volume (patient with BED) the whole blood concentration of the drug will be lower than in a patient with a very poor volume distribution (patient with AN).
This pilot clinical research report is weakened by several limitations. First it has been carried out in a "real world" outpatients setting with all the difficulties associated with the organization of the collection of samples. Indeed, although we did our best to avoid possible mistakes, we had to rely merely on patients’ compliance in taking the drugs. Further limitations include devices and analyses costs which prevented us from the possibility of having a larger sample size, and the heterogeneity of the BMI included, of the drugs taken and of the medical comorbidities of patients’. Our next aim will be to perform further studies in order to obtain an enriched sample size, a better standardization to reduce ?t variations among the sample of patients, and to correlate the clinical outcome with the plasmatic antidepressant concentrations. The evaluation of whole blood and salivary concentrations of antidepressants in underweight or obese patients with ED allows us to formulate hypotheses and pharmacokinetic conjectures on the particular ineffectiveness of these drugs in ED patients.
In this context, the present report and the thesis that derives from it intend to focus attention also on the need for further and more in-depth studies, which will explore the pharmacokinetic and pharmacodynamic mechanisms, as well as the neurobiological reasons underlying the strong resistance of the Disorders of the Food behaviour to drug treatment and/or the prevalence of side effects that may affect the compliance to the treatments.
More studies in this direction are necessary to improve the global and multidisciplinary management of these disorders, characterized by the highest mortality rate in the field of psychiatric diseases and which today represent a real mental health emergency, as well as a problem of public health [56].
Author Disclosure
This study did not have funding sources.
Author Contribution
Panariello F. Analized the data, managed the literature searches and analyses and wrote the first draft of the manuscript; Mastellari T. , Wrote the protocol and collected the data; di Gianni A. Wrote the protocol and collected the data; Fornasari F. Managed the literature searches and helped Panariello F to write; Protti M. Processed, analysed the samples and undertook the statistical analysis; Mercolini L. Processed, analysed the samples and undertook the statistical analysis; De Ronchi D. Designed the study and wrote the first draft of the manuscript; Atti AR Designed the study, wrote the first draft of the manuscript and undertook the statistical analysis; All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, DSM-5 (2013).
- NICE guideline. Eating disorders: recognition and treatment [Internet] (2017).
- McElroy SL, Guerdjikova AI, Mori N, et al. Psychopharmacologic Treatment of Eating Disorders: Emerging Findings. Current Psychiatry Reports (2015).
- Claudino AM, Hay P, Lima MS, et al. Antidepressants for anorexia nervosa. Cochrane Database Syst Rev (2006).
- Davis H, Attia E. Pharmacotherapy of eating disorders. Current Opinion in Psychiatry 30 (2017): 452.
- Himmerich H, Treasure J. Psychopharmacological advances in eating disorders. Expert Rev Clin Pharmacol; 11 (2018): 95-108.
- Jackson CW, Cates M, Lorenz R. Pharmacotherapy of Eating Disorders. Nutrition in Clinical Practice 25 (2010): 143-159.
- Marvanova M, Gramith K. Role of antidepressants in the treatment of adults with anorexia nervosa. Ment Health Clin 8 (2018): 127-137.
- Alañón Pardo MDM, Ferrit Martín M, Calleja Hernández MÁ, et al. Adherence of psychopharmacological prescriptions to clinical practice guidelines in patients with eating behavior disorders. Eur J Clin Pharmacol 73 (2017): 1305-1313.
- Garner DM, Anderson ML, Keiper CD, et al. Psychotropic medications in adult and adolescent eating disorders: clinical practice versus evidence-based recommendations. Eat Weight Disord 21 (2016): 395-402.
- Mizusaki K, Gih D, LaRosa C, et al. Psychotropic usage by patients presenting to an academic eating disorders program. Eat Weight Disord 23 (2018): 769-774.
- Kazdin AE, Fitzsimmons?Craft EE, Wilfley DE. Addressing critical gaps in the treatment of eating disorders. International Journal of Eating Disorders 50 (2017): 170-189.
- Phillipou A, Rossell SL, Castle DJ. The neurobiology of anorexia nervosa: a systematic review. Aust N Z J Psychiatry 48 (2014): 128-152.
- Kaye W, Gendall K, Strober M. Serotonin neuronal function and selective serotonin reuptake inhibitor treatment in anorexia and bulimia nervosa. Biol Psychiatry 44 (1998): 825-838.
- Barbarich NC, McConaha CW, Halmi KA, et al. Use of nutritional supplements to increase the efficacy of fluoxetine in the treatment of anorexia nervosa. International Journal of Eating Disorders 35 (2004): 10-15.
- Duriez P, Ramoz N, Gorwood P, et al. A Metabolic Perspective on Reward Abnormalities in Anorexia Nervosa. Trends Endocrinol Metab 30 (2019): 915-928.
- Paulzen M, Haen E, Stegmann B, et al. Body mass index (BMI) but not body weight is associated with changes in the metabolism of risperidone; A pharmacokinetics-based hypothesis. Psychoneuroendocrinology 73 (2016): 9-15.
- Sigurdsson HP, Hefner G, Ben-Omar N, et al. Steady-state serum concentrations of venlafaxine in patients with late-life depression. Impact of age, sex and BMI. J Neural Transm (Vienna) 122 (2015): 721-729.
- Uher R, Mors O, Hauser J, et al. Body weight as a predictor of antidepressant efficacy in the GENDEP project. J Affect Disord 118 (2009): 147-154.
- Unterecker S, Deckert J, Pfuhlmann B. No influence of body weight on serum levels of antidepressants. Ther Drug Monit 33 (2011): 730-734.
- Mercolini L, Saracino MA, Protti M. Current advances in biosampling for therapeutic drug monitoring of psychiatric CNS drugs. Bioanalysis 7 (2015): 1925-1942.
- Protti M, Mandrioli R, Mercolini L. Tutorial: Volumetric absorptive microsampling (VAMS). Analytica Chimica Acta 1046 (2019): 32-47.
- Baumann P, Hiemke C, Ulrich S, et al. Arbeitsge-meinschaft fur neuropsychopharmakologie und pharmakopsychiatrie. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry 37 (2004): 243-265.
- Hiemke C, Bergemann N, Clement H, et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 51 (2018): 9-62.
- Mandrioli R, Mercolini L, Saracino MA, et al. Selective serotonin reuptake inhibitors (SSRIs): therapeutic drug monitoring and pharmacological interactions. Curr Med Chem 19 (2012): 1846-1863.
- Bauer L. Applied Clinical Pharmacokinetics, 3rd ed. New York, McGraw-Hill (2014).
- Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 82 (2007): 87-96.
- Burke MJ, Preskorn SH. Therapeutic drug monitoring of antidepressants: cost implications and relevance to clinical practice. Clin Pharmacokinet 37 (1999): 147-165.
- Lundmark J, Bengtsson F, Nordin C, et al. Therapeutic drug monitoring of selective serotonin reuptake inhibitors influences clinical dosing strategies and reduces drug costs in depressed elderly patients. Acta Psychiatr Scand 101 (2000): 354-359.
- Ostad Haji E, Mann K, Dragicevic A, et al. Potential cost-effectiveness of therapeutic drug monitoring for depressed patients treated with citalopram. Ther Drug Monit 35 (2013): 396-401.
- Simmons SA, Perry PJ, Rickert ED, et al. Cost-benefit analysis of prospective pharmacokinetic dosing of nortriptyline in depressed inpatients. J Affect Disord 8 (1985): 47-53.
- Delahaye L, Dhont E, De Cock P, et al. Volumetric absorptive microsampling as an alternative sampling strategy for the determination of paracetamol in blood and cerebrospinal fluid. Anal Bioanal Chem 411 (2019): 181-191.
- Kip AE, Kiers KC, Rosing H, et al. Volumetric absorptive microsampling (VAMS) as an alternative to conventional dried blood spots in the quantification of miltefosine in dried blood samples. J Pharm Biomed Anal 135 (2017): 160-166.
- Kok MGM, Fillet M. Volumetric absorptive microsampling: Current advances and applications. J Pharm Biomed Anal 147 (2018): 288-296.
- Mercolini L, Protti M, Catapano MC, et al. LC-MS/MS and volumetric absorptive microsampling for quantitative bioanalysis of cathinone analogues in dried urine, plasma and oral fluid samples. J Pharm Biomed Anal 123 (2016).
- Li R-R, Sheng X-Y, Ma L-Y, et al. Saliva and Plasma Monohydroxycarbamazepine Concentrations in Pediatric Patients With Epilepsy. Ther Drug Monit 38 (2016): 365-370.
- Dwivedi R, Singh M, Kaleekal T, et al. Concentration of antiepileptic drugs in persons with epilepsy: a comparative study in serum and saliva. Int J Neurosci 126 (2016): 972-978.
- Baumann P, Tinguely D, Koeb L, et al. On the relationship between free plasma and saliva amitriptyline and nortriptyline. Int Pharmacopsychiatry 17 (1982): 136-146.
- Dwivedi R, Gupta YK, Singh M, et al. Correlation of saliva and serum free valproic acid concentrations in persons with epilepsy. Seizure 25 (2015): 187-190.
- Patteet L, Maudens KE, Morrens M, et al. Determination of Common Antipsychotics in Quantisal-Collected Oral Fluid by UHPLC-MS/MS: Method Validation and Applicability for Therapeutic Drug Monitoring. Ther Drug Monit 38 (2016): 87-97.
- Khan A, Schwartz KA, Kolts RL, et al. BMI, sex, and antidepressant response. J Affect Disord 99 (2007): 101-106.
- Kloiber S, Ising M, Reppermund S, et al. Overweight and Obesity Affect Treatment Response in Major Depression. Biological Psychiatry 62 (2007): 321-326.
- Anoshkina Y, Costas-Rodríguez M, Vanhaecke F. Iron isotopic analysis of finger-prick and venous blood by multi-collector inductively coupled plasma-mass spectrometry after volumetric absorptive microsampling. J Anal At Spectrom 32 (2017): 314-321.
- De Kesel PMM, Lambert WE, Stove CP. Does volumetric absorptive microsampling eliminate the hematocrit bias for caffeine and paraxanthine in dried blood samples? A comparative study. Anal Chim Acta 881 (2015): 65-73.
- Heussner K, Rauh M, Cordasic N, et al. Adhesive blood microsampling systems for steroid measurement via LC-MS/MS in the rat. Steroids 120 (2017): 1-6.
- Kopp M, Rychlik M. Assessing Volumetric Absorptive Microsampling Coupled with Stable Isotope Dilution Assay and Liquid Chromatography-Tandem Mass Spectrometry as Potential Diagnostic Tool for Whole Blood 5-Methyltetrahydrofolic Acid. Front Nutr 4 (2017): 9.
- Marahatta A, Megaraj V, McGann PT, et al. Stable-Isotope Dilution HPLC-Electrospray Ionization Tandem Mass Spectrometry Method for Quantifying Hydroxyurea in Dried Blood Samples. Clin Chem 62 (2016): 1593-1601.
- Nys G, Gallez A, Kok MGM, et al. Whole blood microsampling for the quantitation of estetrol without derivatization by liquid chromatography-tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis 140 (2017): 258-265.
- Protti M, Rudge J, Sberna AE, et al. Dried haematic microsamples and LC-MS/MS for the analysis of natural and synthetic cannabinoids. J Chromatogr B Analyt Technol Biomed Life Sci 1045 (2017): 77-86.
- Chen G, Højer A-M, Areberg J, et al. Vortioxetine: Clinical Pharmacokinetics and Drug Interactions. Clin Pharmacokinet 57 (2018): 673-686.
- Ostad Haji E, Hiemke C, Pfuhlmann B. Therapeutic drug monitoring for antidepressant drug treatment. Curr Pharm Des 18 (2012): 5818-5827.
- Kotlyar M, Carson SW. Effects of obesity on the cytochrome P450 enzyme system. Int J Clin Pharmacol Ther 37 (1999): 8-19.
- Rahmioglu N, Heaton J, Clement G, et al. Genetic epidemiology of induced CYP3A4 activity. Pharmacogenetics and Genomics 21 (2011): 642.
- Tomankova V, Anzenbacher P, Anzenbacherova E. Effects of obesity on liver cytochromes P450 in various animal models. Biomedical Papers 161 (2017): 144-151.
- Unterecker S, Deckert J, Pfuhlmann B. No In?uence of Body Weight on Serum Levels of Antidepressants. Ther Drug Monit 33 (2011): 5.
- EpiCentro - Istituto Superiore di Sanità. Anoressia e bulimia (2019).


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
