Tocilizumab-Induced Limbic Encephalitis: Another Adverse Effect of Disrupted IL-6 Signalling?
Sina Helms1, Christoph Müller1, Nico Melzer1,2*
1Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Albert-Schweitzer-Campus 1, 48149 Münster, Germany
2Department of Neurology, Heinrich-Heine University of Düsseldorf, Moorenstraße 5, 40225 Düsseldorf, Germany
*Corresponding Author: Nico Melzer, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Albert-Schweitzer-Campus 1, 48149 Münster, Germany
Received: 01 September 2021; Accepted: 16 September 2021; Published: 21 October 2021
Article Information
Citation: Sina Helms, Christoph Müller, Nico Melzer. Tocilizumab-Induced Limbic Encephalitis: Another Adverse Effect of Disrupted IL-6 Signalling?. Archives of Clinical and Medical Case Reports 5 (2021): 704-709.
View / Download Pdf Share at FacebookKeywords
<p>Tocilizumab; Immunoglobin; Anti-human interleukin-6 receptor; Monoclonal antibody</p>
Article Details
1. Introduction
Tocilizumab is a humanized, immunoglobin G1κ (IgG1κ) anti-human interleukin-6 receptor (anti-IL-6R) monoclonal antibody (mAb). Binding of IL-6 to the transmembrane (“cis-signaling”) or soluble (“trans-signaling”) form of IL-6R causes homodimerization of the ubiquitously expressed gp130 protein and intracellular signaling via the Jak/Stat pathway [1]. This signaling cascade leads – among others – to pleiotropic effects on the adaptive immune system: IL-6 increases the Th17/Treg balance [1], induces differentiation of CD8+ T-cells into cytotoxic T-cells, and activates B-cells into antibody-producing plasma cells [1]. Selective inhibition of IL-6-IL-6R binding via tocilizumab is currently approved for the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis, giant cell arteritis, Castleman's disease, and cytokine release syndromes associated with CAR-T cell therapies. Positive treatment effects have also been reported in neuromyelitis optica spectrum disorder [2-6] and autoimmune encephalitis [7, 8]. Common adverse effects of tocilizumab include infections, liver dysfunction, diverticulitis and perforation, haematological and metabolic abnormalities, and infusion reactions.
2. Case Report
Here, we present the case of an otherwise healthy 74-year-old male patient who received tocilizumab (162 mg s.c. once per week) together with oral prednisolone (50 mg/day) for giant cell arteritis. 8 weeks after treatment initiation, the patient was admitted to our hospital suspected for having a first epileptic seizure. The patient showed decreased levels of consciousness without responsiveness and a subsequent confusional state with severe anterograde but also retrograde amnesia. MRI at baseline showed symmetric T2-/FLAIR signal hyperintensity and swelling of both hippocampi without Gd-enhancement (Figure 1). Repeated surface EEG recordings revealed generalized slowing of background activity with additional regional right anterior temporal slowing and sharp transients but no clear epileptic activity. Repeated CSF analysis including cell-count, protein concentration, serum-CSF ratios for albumin and immunoglobulin M, -A, and G, isoelectric focussing (9, type 1 pattern; Figure 1) as well as flow cytometry analysis of peripheral blood and CSF cells [10] was normal. A repeated extensive panel of anti-neural autoantibodies using a combination of tissue- and cell-based assays and immunoblots in serum and CSF was normal. Repeated screening for systemic markers of autoimmunity and viral, bacterial and fungal infection remained unremarkable. CSF Tau-protein levels at baseline were increased (2777 pg/ml) with normal amyloid β levels (1618 pg/ml; Figure 1) indicating acute neuronal damage. Indeed, MRI follow-up after 1 week demonstrated incipient bilateral hippocampal atrophy (Figure 1).
A potential adverse effect of tocilizumab treatment was suspected [11-13]. Tocilizumab was stopped immediately and removed (together with potentially elevated levels of cytokines) from the circulation via 5 cycles of plasma exchange together with methylprednisolone pulse therapy (5 x 1g/d). Immunosuppressive treatment was continued with methotrexate (15 mg/week) and oral prednisolone (50 mg/day). Anticonvulsive treatment was performed with lacosamide (200 mg/day).
Upon follow-up 6 and 18 weeks after disease onset, the patient remained seizure-free on constant anticonvulsive medication. Memory performance deteriorated between week 1 and week 6 and did not change significantly afterwards. EEG alterations went back to normal. Hippocampal T2-/FLAIR signal intensity decreased with no additional volume loss on MRI. Consistently, CSF Tau-protein levels went back to normal while amyloid β levels stayed normal (Figure 1).
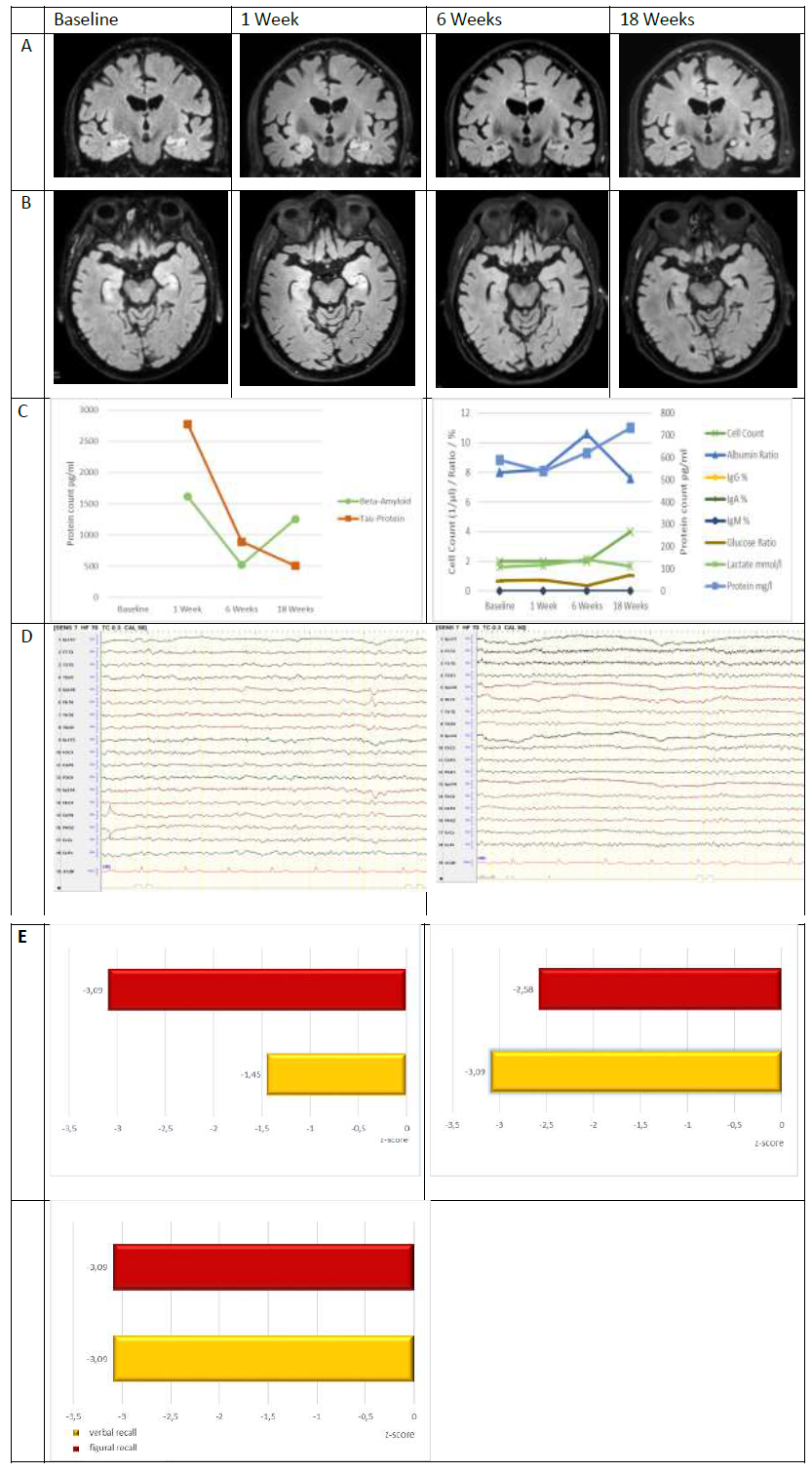
Figure 1: Summary of MRI, CSF, EEG and neuropsychological findings, baseline vs. 1 Week vs. 6 Weeks vs. 18. Weeks. A: MRI scan, coronal FLAIR sequence; B: MRI scan, axial FLAIR sequence; baseline: symmetric T2-/FLAIR signal hyperintensity and swelling of both hippocampi without Gd-enhancement, 1 Week: incipient bilateral hippocampal atrophy, 6 / 18 Weeks: Decreasing T2/FLAIR signal hyperintensity with no additional volume loss C: CSF findings regarding amyloid β and tau proteins as well as cell-count, protein-, lactate-, and glucose concentrations and albumin ratio; D: Routine-EEG findings (longitudinal bipolar montage), E: Z-scores for figural and verbal memory function assessed with CERAD [14].
3. Discussion
Our case is consistent with acute tocilizumab-induced hippocampal inflammation or edema with neuronal cell death without evidence of an overt underlying cerebral or systemic cellular immune response stopped by immediate removal of tocilizumab (and cytokines) via plasma exchange. Similiar cases of more wide-spread tocilizumab-associated (menigo-)encephalitis with subsequent atrophy involving both cerebral grey and white matter probably due to delayed diagnosis and lack of specific treatment (plasma exchange) were described [11-13].
Tocilizumab binding to IL-6R dramatically increases the level of free IL-6 [11-13, 15]. As long as free tocilizumab is available (at high doses of tocilizumab), IL-6R is saturated with tocilizumab and IL-6 signalling is completely inhibited. However, if all tocilizumab is bound to IL-6R (at low doses of tocilizumab) excessive IL-6 may bind to free IL-6R and exert intense proinflammatory signalling [15]. This may be associated with acute and chronic meningoencephalitis [16]. Consistently, cerebral inflammation due to dysregulated cytokine signalling is known to cause demyelinating CNS disorders under treatment with TNFa-inhibitors [11].
Hence, after careful exclusion of concurrent causes, treatment-induced acute (menigo-)encephalitis should be suspected in patients under tocilizumab (and other anti-cytokine) treatment that present with acute encephalopathy with and without seizures. Treatment approaches should include removal of tocilizumab (and potentially excessive cytokine levels) via plasma exchange.
References
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6 (2014): a016295.
- Zhang C, Zhang M, Qiu W, et al. TANGO Study Investigators. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (TANGO): an open-label, multicentre, randomised, phase 2 trial. Lancet Neurol 19 (2020): 391-401.
- Ayzenberg I, Kleiter I, Schröder A, et al. Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti-CD20 therapy. JAMA Neurol 70 (2013): 394-397.
- Ringelstein M, Ayzenberg I, Harmel J, et al. Long-term Therapy With Interleukin 6 Receptor Blockade in Highly Active Neuromyelitis Optica Spectrum Disorder. JAMA Neurol 72 (7): 756-763.
- Araki M, Matsuoka T, Miyamoto K, et al. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology 82 (2014): 1302-1306.
- Kieseier BC, Stüve O, Dehmel T, et al. Disease amelioration with tocilizumab in a treatment-resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol 70 (2013): 390-393.
- Lee WJ, Lee ST, Moon J, et al. Tocilizumab in Autoimmune Encephalitis Refractory to Rituximab: An Institutional Cohort Study. Neurotherapeutics 13 (2016): 824-832.
- Randell RL, Adams AV, Van Mater H. Tocilizumab in Refractory Autoimmune Encephalitis: A Series of Pediatric Cases. Pediatr Neurol 86 (2018): 66-68.
- Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 184 (2001): 101-122.
- Golombeck KS, Bönte K, Mönig C, et al. Evidence of a pathogenic role for CD8 (+) T cells in anti-GABAB receptor limbic encephalitis. Neurol Neuroimmunol Neuroinflamm 3 (2016): e232.
- Fockaert N, Goffin K, Demaerel P, et al. Infliximab-associated autoimmune limbic encephalitis: a case report. Acta Neurol Belg 115 (2015): 161-163.
- Kobayashi K, Okamoto Y, Inoue H, et al. Leukoencephalopathy with cognitive impairment following tocilizumab for the treatment of rheumatoid arthritis (RA). Intern Med. 48 (2009): 1307-1309.
- Yamaguchi Y, Furukawa K, Yamamoto T, et al. Multifocal encephalopathy and autoimmune-mediated limbic encephalitis following tocilizumab therapy. Intern Med 53 (2014): 879-882.
- Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 44 (1994): 609-614.
- Nishimoto N, Terao K, Mima T, et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112 (2008): 3959-3964.
- Salsano E, Rizzo A, Bedini G, Bernard L, et al. An autoinflammatory neurological disease due to interleukin 6 hypersecretion. J Neuroinflammation 10 (2013): 29.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
