Two Recovered COVID-19 Cases Testing Positive Again for SARS-CoV-2 After Discharge in Hanoi, Vietnam
Pham Ngoc Thach#, Nguyen Tuan Anh*#, Nguyen Viet Hang, Le Van Duyet*
#Authors contributed equally
National Hospital for Tropical Diseases, Hanoi, Vietnam
*Corresponding Authors: Dr. Le Van Duyet, National Hospital for Tropical Diseases, Hanoi, Vietnam
Dr. Nguyen Tuan Anh, National Hospital for Tropical Diseases, Hanoi, Vietnam
Received: 22 June 2020; Accepted: 17 July 2020; Published: 03 September 2020
Article Information
Citation: Pham Ngoc Thach, Nguyen Tuan Anh, Nguyen Viet Hang, Le Van Duyet. Two Recovered COVID-19 Cases Testing Positive Again for SARS-CoV-2 After Discharge in Hanoi, Vietnam. Archives of Clinical and Medical Case Reports 4 (2020): 766-773.
View / Download Pdf Share at FacebookAbstract
Vietnam has successfully controlled the first waves of SARS-CoV-2 and has seen no community transmission since 15 April. One of the keys to this success is consistent quarantine of individuals who have been in contact with suspected cases or who are entering Vietnam from an endemic country. Reliable diagnostics to end isolation of patients and quarantine of individuals at risk are an essential component of this strategy. In this study we report two cases of who were discharged after testing negative twice for SARS-CoV-2 and then tested positive again. These are the first cases of virological relapse detected in Vietnam.
Keywords
<p>SARS-CoV-2; COVID-19; Viorological relapse; Tropical Diseases</p>
Article Details
1. Introduction
The outbreak of COVID-19 was first reported from Wuhan, China in December 2019, within months COVID-19 spread globally and a pandemic was formally declared by WHO in March [1]. On 26 May, the total number of infections worldwide was over 5 and half million with more than 350,000 deaths. Vietnam detected its first case of COVID - 19 on 23 January 2020 and has been successful in controlling the national outbreak through a rapid and consistent response consisting of strict quarantining based on exposure and entry into Vietnam from an endemic country. There has been no community transmission since 15 April [2]. On 23 May, there were a total of 325 positive cases with SARS-CoV-2 detected, including 267 cases who were discharged according to the standards of the Ministry of Health and no deaths [3].
Among these 267 discharged cases, there were 2 who tested positive again during the 2 weeks of recommended post-discharge self quarantine at home. This phenomenon of virological relapse has also been reported from Korea, China and Japan [4-8]. It was reported by local health authorities in Guangdong, China that the proportion of patients with virological relapse after clinical cure and testing negative was 14% of all patients discharged. Currently, there is no evidence that this viorological relapse is associated with actrively replicating virus or infectivity. Here, we describe the two first cases with virological relapse in Vietnam from a community cluster in Vinh Phuc province [9, 10].
2. Case Presentation
2.1 Case 01
The patient was a 24-year-old female from Son Loi Village, Binh Xuyen District, Vinh Phuc Province. On 17 January 2020, she returned to Vietnam from Wuhan. After returning home, the patient developed fever (> 38 oC), sore throat and dry cough. Between 27 January to 2 March 2020, she was admitted to the National Hospital of Tropical Diseases (Dong Anh Campus) with a definite diagnosis of COVID-19 (Figure 1). The patient was one of the first three cases in Vietnam. On 2 March 2020, the patient was clinically recovered and had tested negative twice for SARS-CoV-2 using realtime RT-PCR test [11] and the patient was eligible for discharge from hospital according to the Ministry of Health standards. After being discharged, the patient was required to self-quarantine at home for 14 days followed by repeat testing.
A sample taken on 4 March 2020 (two days after discharge from the hospital) tested positive again. On 8 March 2020, the patient was isolated again at the National Hospital for Tropical Diseases with no clinical symptoms, normal laboratory tests and no lesions on chest X-ray and computerized tomography (Figure 2). Urine, rectal swab and stool were collected and tested by realtime RT-PCR but were all negative. Only oropharyngeal swabs remained positive on 12, 18, and 20 March 2020 (Table 1). This was followed by 4 negative times and then continued positive again on 6/4/2020. Currently patients continue to be isolated for follow-up testing.
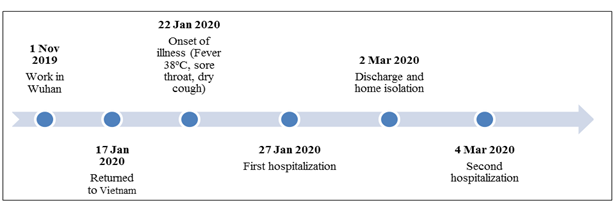
Figure 1: Infection timeline of patient 01.
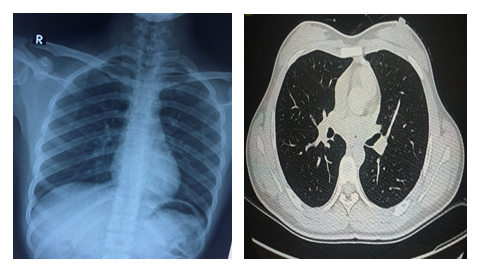
Figure 2: X-ray and computed tomography of the patient without injury.
|
Time |
Days after illness |
realtime RT-PCR |
Ct value E gene |
|
23 Jan |
0 |
+ |
30.16 |
|
25 Jan |
2 |
+ |
32.64 |
|
30 Jan |
8 |
+ |
33.27 |
|
7 Feb |
15 |
+ |
34.19 |
|
08 Feb |
16 |
+ |
36.25 |
|
29 Feb |
37 |
- |
- |
|
2 Mar |
39 |
- |
- |
|
4 Mar |
41 |
+ |
37.25 |
|
8 Mar |
45 |
+ |
36.76 |
|
12 Mar |
49 |
+ |
36.80 |
|
18 Mar |
55 |
+ |
37.35 |
|
20 Mar |
57 |
+ |
37.72 |
|
29 Mar |
66 |
- |
- |
|
31 Mar |
68 |
- |
- |
|
2 Mar |
70 |
- |
- |
|
4 Mar |
72 |
- |
- |
|
6 Apr |
74 |
+ |
36.51 |
|
8 Apr |
76 |
+ |
37.02 |
|
10 Apr |
78 |
+ |
37.61 |
Table 1: SARS-CoV-2 realtime RT-PCR test variation for patient 01.
2.2 Case 02
The patient was a 49-year-old female residing in Son Loi Village, Binh Xuyen District, Vinh Phuc Province. She is the mother of case 1 and lives in the same house. The samples of pancreatic and pharyngeal swab were collected because of her contact history with case 1 and sent to CDC Ha Noi for realtime RT-PCR and were positive for SARS-CoV-2. Between 6 February 2020 and 6 March 2020, the patient was admitted to the Medical Center of Binh Xuyen District, Vinh Phuc Province (Figure 3). The patient was discharged after clinical recovery and testing negative twice on 20 and 22 January and was asked to self-quarantine at home for 14 more days according to MoH regulations. At home, she had close contact with case 1. On 8 March 2020, she and case 1 were readmitted and isolated at the National Hospital for Tropical Diseases. She had no clinical symptoms, normal laboratory tests and no abnormalities on chest X-ray and computerized tomography (Figure 4). The oropharyngeal swabs were collected and performed realtime RT-PCR for SARS-CoV-2, the results revealed very complicated (Table 2). Currently patients continue to be isolated for follow-up testing.
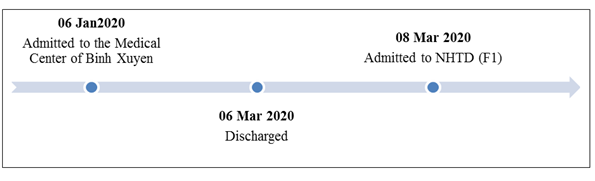
Figure 3: Infection timeline of patient 02.
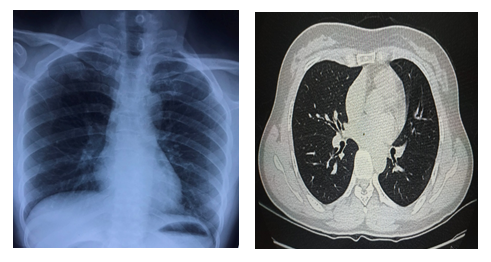
Figure 4: X-ray and computed tomography of the patient without injury.
|
Time |
Days after illness |
realtime RT-PCR |
Ct value E gene |
|
6 Jan |
0 |
+ |
26.64 |
|
10 Jan |
4 |
+ |
28.28 |
|
18 Jan |
12 |
+ |
35.35 |
|
20 Jan |
14 |
- |
- |
|
22 Jan |
16 |
- |
- |
|
26 Jan |
20 |
- |
- |
|
2 Mar |
25 |
- |
- |
|
7 Mar |
29 |
- |
- |
|
8 Mar |
30 |
+ |
36.32 |
|
11 Mar |
34 |
+ |
37.24 |
|
17 Mar |
39 |
- |
- |
|
20 Mar |
42 |
+ |
37.92 |
|
22 Mar |
44 |
+ |
37.12 |
Table 2: SARS-CoV-2 realtime RT-PCR test variation for patient 02.
3. Discussion
SARS-CoV-2 was recognized as the 7th coronavirus that can cause disease in humans after 229E, OC43, NL63, HKU1, SARS-CoV and MERS-CoV. The genome sequence of SARS-CoV-2 was reported to be 96% homologous compared to coronaviruses detected in horseshoe bats and approximately 79.5% similar to the SARS-CoV [12]. The disease spectrum of COVID-19 ranges from asymptomatic in a large proportion of patients to severe and fatal, especially among the elderly and those with underlying illness.
SARS-CoV-2 has an estimated basic reproductive number (R0) of between 2.2 and 3.6 and the main route of transmission is through droplets from an infected individuals cough or sneeze within a range of 1-2m or through indirect contact with the secretions of an infected person through objects or surroundings [13-16]. Recent findings from Shenzhen, China showed the presence of coronavirus RNA in the feces of patients infected with SARS-CoV-2, suggestsing the virus could also be transmitted through the gastrointestinal tract. Epidemiological surveys show that patients with COVID-19 are still the main source of infection [17]. Asymptomatic carriers may also be sources of infection [18, 19]. Therefore, the diagnosis, management and treatment of infected patients are very important.
According to the guidelines issued by the Ministry of Health of Vietnam, patients discharged from hospital must meet the following criteria: (1) the body temperature returned to normal for more than 3 days; (2) improved clinical symptoms, good general status, stable vital signs, normal organ function, blood test returning to normal, improved chest X-ray; (3) at least two consecutive SARS-CoV-2 tests negative in respiratory swabs 24 hours apart [4]. The two cases reported above met the requirements and were discharged, but soon found positive again. This raises questions about the reliability of the current standards for discharge to prevent further community transmission, and about the risk of transmission from asymptomatic recovered patients with virological relapse. However, viral culture results from patients with virological relapse in Vietnam have so far remained negative.
During infection with SARS-CoV-2 the human body produces neutralizing antibodies and patients are considered immune to re-infection after clinical recovery. Both patients reported no post-discharge exposure to or suspected COVID-19 after being discharged from the hospital. They continued to have close contact during the post-discharge period of self-quarantine and may have infected each other. However, there is no evidence to date that patients with virological relapse of SARS-CoV-2 could be infectious and viral culture of detection of subgenomic RNA suggesting active replication of samples from similar patients in Korea and Singapore have remained negative [20, 21].
Real-time RT-PCR results in general and also for SARS-CoV-2 can be either negative or positive in a stochastic manner when the copy number in the tested sample is close to the detection limit. Results may depend on: (1) the type of specimen collected; (2) the way the specimen was collected; (3) previous use of antiviral drugs; (4) sensitivity of testing techniques; (5) mutations in the target sequence. It has been hypothesized that the late detection of viral RNA in COVID-19 is a result of recovery of lung tissue and excretion of damaged cells and tissue that still contain viral RNA remnants but no actively replicating virus [22].
The most commonly used sampling method are nasopharyngeal and throat swabs. However, the main site of infection with SARS-CoV-2 is the lower respiratory tract, and a certain amount of virus is required for a test to be positive. Accordingly, collection of sputum or lower respiratory tract specimens from intubated patients may have a higher yield. Theoretically, mutations in the target sequence can also lead to negative results, and therefore, use of multiple target genes may also increase diagnostic yield and reliability.
4. Conclusion
Patients met the standards for recovery and were discharged according to the guideline of the Ministry of Health of Vietnam can still re-positive when tested by realtime RT-PCR for SARS-CoV-2. It is uncertain whether these patients may still represent a source of infection. The criteria for cure, the accuracy of the test as well as the management of patients after discharge should be re-evaluated. Above are the initial results that we give through two actual cases recorded in Vietnam. It is necessary to have a larger epidemiological study to clarify the basis for making treatment standards, standards for curing disease and hospitalization, and recommendations for epidemic management.
References
- Zhu N, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 382 (2020): 727-733.
- Pham TQ, et al. The first 100 days of SARS-CoV-2 control in Vietnam. medRxiv (2020): 2020.05.12.20099242.
- Ministry of Health, V. COVID-19 situation update. 2020 [cited 2020 2 MAY 2020]; Available from: https://ncov.moh.gov.vn/web/guest/trang-chu.
- Ann J, et al. Clinical characteristics of the recovered COVID-19 patients with re-detectable positive RNA test. medRxiv (2020): 2020.03.26.20044222.
- Ling Y, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 133 (2020): 1039-1043.
- Lan L, et al. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA (2020).
- Qu YM, Kang EM, Cong HY. Positive result of Sars-Cov-2 in sputum from a cured patient with COVID-19. Travel Med Infect Dis 34 (2020): 101619.
- Xing Y, et al. Prolonged presence of SARS-CoV-2 in feces of pediatric patients during the convalescent phase. medRxiv (2020): 2020.03.11.20033159.
- Thanh HN, et al. Outbreak investigation for COVID-19 in northern Vietnam. Lancet Infect Dis (2020).
- Le TQM, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Shedding by Travelers, Vietnam, 2020. Emerg Infect Dis 26 (2020).
- Corman VM, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25 (2020).
- Zhou P, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579 (2020): 270-273.
- Zhang S, et al. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: A data-driven analysis. Int J Infect Dis 93 (2020): 201-204.
- Li Q, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 382 (2020): 1199-1207.
- Wu P, et al. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill 25 (2020).
- Sanche S, et al. High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis 26 (2020).
- Cheung K.S, et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from the Hong Kong Cohort and Systematic Review and Meta-analysis. Gastroenterology (2020).
- Rothe C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med 382 (2020): 970-971.
- Yu P, et al. A Familial Cluster of Infection Associated With the 2019 Novel Coronavirus Indicating Possible Person-to-Person Transmission During the Incubation Period. J Infect Dis 221 (2020): 1757-1761.
- https://www.npr.org/sections/coronavirus-live-updates/2020/04/17/836747242/in-south-korea-a-growing-number-of-covid-19-patients-test-positive-after-recover.
- Chen D, et al. Recurrence of positive SARS-CoV-2 RNA in COVID-19: A case report. Int J Infect Dis 93 (2020): 297-299.
- Li H, et al. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents 55 (2020): 105951.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
