Urgent Surgery for Giant Pancreatic Neuroendocrine Tumor: A Case Report
Giménez Maurel Teresa1, Utrilla Fornals Alejandra1, Serrablo Casaña Leyre3, Trigo Gómez Miguel Angel4, Serrablo Requejo Alejandro2
1General Surgery Department, San Jorge Hospital, Huesca, Spain
2Hepatopancreatic Biliary Surgical Division, Miguel Servet University Hospital, Zaragoza, Spain
3Medicine School, Zaragoza University, Spain
4Pathological Anatomy Department, Miguel Servet University Hospital, Zaragoza, Spain
*Corresponding Author: Dr. Giménez Maurel, General Surgery Department, San Jorge Hospital, Huesca, Spain
Received: 05 May 2020; Accepted: 02 June 2020; Published: 10 July 2020
Article Information
Citation: Giménez Maurel Teresa, Utrilla Fornals Alejandra, Serrablo Casaña Leyre, Trigo Gómez Miguel Angel, Serrablo Requejo Alejandro. Urgent Surgery for Giant Pancreatic Neuroendocrine Tumor: A Case Report. Archives of Clinical and Medical Case Reports 4 (2020): 649-657.
View / Download Pdf Share at FacebookAbstract
Pancreatic neuroendocrine neoplasms (P-NENs) are a heterogeneus group of tumors with a low frequency of occurrence (<1/100.000 patients per year). Their incidence has been increasing in the past decades mainly due to incidental discovery of asymptomatic lesions. Approximately, 60% of patients present metastases during the diagnostic process and the liver is the most frequent location where the methastases present themselves. Even though in 2010, the World Health Organization (WHO) presented the most accepted classification so far, that divided P-NENs into well-differentiated neuroendocrine tumors (NETs) and less well-differentiated neuroendocrine carcinomas (NECs), a universally standardized classification is still needed. Imaging tests such as CT or MRI are frequently used for diagnosis but Octreoscan and further complex techniques including preoperative biopsy are also needed for a successful diagnosis. Surgery is the only possible curative treatment, however added adyuvant chemoterapy is considered to improve survival rates.
We present the case of a 56-year-old patient who suffered from P-NEN and underwent an aggressive surgery with curative intent. We consider the interest given due to the large size of the tumor, its liver metastases, and the correct surgical and oncological results obtained.
Keywords
Neuroendocrin tumors; Pancreatic neoplasms; Radical surgery; Pancreatectomy; Hepatic metastasis
Neuroendocrin tumors articles, Pancreatic neoplasms articles, Radical surgery articles, Pancreatectomy articles, Hepatic metastasis articles
Neuroendocrin tumors articles Neuroendocrin tumors Research articles Neuroendocrin tumors review articles Neuroendocrin tumors PubMed articles Neuroendocrin tumors PubMed Central articles Neuroendocrin tumors 2023 articles Neuroendocrin tumors 2024 articles Neuroendocrin tumors Scopus articles Neuroendocrin tumors impact factor journals Neuroendocrin tumors Scopus journals Neuroendocrin tumors PubMed journals Neuroendocrin tumors medical journals Neuroendocrin tumors free journals Neuroendocrin tumors best journals Neuroendocrin tumors top journals Neuroendocrin tumors free medical journals Neuroendocrin tumors famous journals Neuroendocrin tumors Google Scholar indexed journals tumors articles tumors Research articles tumors review articles tumors PubMed articles tumors PubMed Central articles tumors 2023 articles tumors 2024 articles tumors Scopus articles tumors impact factor journals tumors Scopus journals tumors PubMed journals tumors medical journals tumors free journals tumors best journals tumors top journals tumors free medical journals tumors famous journals tumors Google Scholar indexed journals Neuroendocrin articles Neuroendocrin Research articles Neuroendocrin review articles Neuroendocrin PubMed articles Neuroendocrin PubMed Central articles Neuroendocrin 2023 articles Neuroendocrin 2024 articles Neuroendocrin Scopus articles Neuroendocrin impact factor journals Neuroendocrin Scopus journals Neuroendocrin PubMed journals Neuroendocrin medical journals Neuroendocrin free journals Neuroendocrin best journals Neuroendocrin top journals Neuroendocrin free medical journals Neuroendocrin famous journals Neuroendocrin Google Scholar indexed journals Pancreatic neoplasms articles Pancreatic neoplasms Research articles Pancreatic neoplasms review articles Pancreatic neoplasms PubMed articles Pancreatic neoplasms PubMed Central articles Pancreatic neoplasms 2023 articles Pancreatic neoplasms 2024 articles Pancreatic neoplasms Scopus articles Pancreatic neoplasms impact factor journals Pancreatic neoplasms Scopus journals Pancreatic neoplasms PubMed journals Pancreatic neoplasms medical journals Pancreatic neoplasms free journals Pancreatic neoplasms best journals Pancreatic neoplasms top journals Pancreatic neoplasms free medical journals Pancreatic neoplasms famous journals Pancreatic neoplasms Google Scholar indexed journals Radical surgery articles Radical surgery Research articles Radical surgery review articles Radical surgery PubMed articles Radical surgery PubMed Central articles Radical surgery 2023 articles Radical surgery 2024 articles Radical surgery Scopus articles Radical surgery impact factor journals Radical surgery Scopus journals Radical surgery PubMed journals Radical surgery medical journals Radical surgery free journals Radical surgery best journals Radical surgery top journals Radical surgery free medical journals Radical surgery famous journals Radical surgery Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals kidney articles kidney Research articles kidney review articles kidney PubMed articles kidney PubMed Central articles kidney 2023 articles kidney 2024 articles kidney Scopus articles kidney impact factor journals kidney Scopus journals kidney PubMed journals kidney medical journals kidney free journals kidney best journals kidney top journals kidney free medical journals kidney famous journals kidney Google Scholar indexed journals Hepatic metastasis articles Hepatic metastasis Research articles Hepatic metastasis review articles Hepatic metastasis PubMed articles Hepatic metastasis PubMed Central articles Hepatic metastasis 2023 articles Hepatic metastasis 2024 articles Hepatic metastasis Scopus articles Hepatic metastasis impact factor journals Hepatic metastasis Scopus journals Hepatic metastasis PubMed journals Hepatic metastasis medical journals Hepatic metastasis free journals Hepatic metastasis best journals Hepatic metastasis top journals Hepatic metastasis free medical journals Hepatic metastasis famous journals Hepatic metastasis Google Scholar indexed journals
Article Details
1. Introduction
Neuroendocrin tumors were firstly described by Oberndorfer S. in 1907 and after that, P-NENs were described in 1927 by Wilder et al. [1-3]. Neurendocrine neoplasms are a heterogeneous group of tumors characterized by their ability to synthesize and secrete hormones, for that reason, they are frecuently separated into two different categories: functioning (hormone secreting) and nonfunctioning (50-70%) neoplasms [2-5]. Even if neuroendocrine neoplasms can arise in most organs, the lungs (25%) and the gastrointestinal tract (60%) are the most frequent locations. As they have multiple clinical and biological manifestations, some of them are unique to the point of origin while others are shared regardless of where they arose [3-7, 9-12]. P-NENs are said to be very infrequent, representing 2-3% of all the pancreatic neoplasms and they have an estimated prevalence of ≤1 cases per 100,000 individuals per year [1-3, 12]. Only a 30% of P-NENs are functioning tumors, mainly insulin or gastrin secreting ones [1, 7]. Most of P-NENs are sporadic, but they can be associated with some hereditary syndromes, such as multiple endocrine neoplasia type I (MEN1), von Hippel-Lindau (VHL) syndrome, neurofibromatosis type I (NF1), and tuberous sclerosis [1, 2].
In the last decades, an increase in P-NENs incidence has been observed, probably due to the improvement of imaging tests, most of them carried out by other pathologies [2-5, 7-10, 12]. The WHO classification was published in 2010; it categorizes NENs into three different groups based on the Ki-67 proliferation index and mitotic count. While NET G1 and NET G2 are presented as well-differentiated endocrine tumors, NEC G3 represents a poorly differentiated endocrine carcinoma/small cell carcinoma [3-6, 9]. As PNENs are frecuently asymptomatic and slow growth tumors, more than 60% of patients are diagnosed when symptoms of local compression or metastatic disease appear, and the liver is the most frequent place of methastases presentation [1, 2, 6, 10]. The potentially curative treatment depends on the type of tumor and its location, but it usually includes surgery (as long as the tumor is resectable) accompanied by adjuvant chemotherapy (for NET G2 and NEC G3), or somatostatin analogues and targeted therapies [2, 5, 7, 12].
Although the role of surgery in the treatment of P-NENs still debated due to their high rate of recurrence [6]. Recent studies show that radical surgery for metastatic P-NENs provides similar survival rates to the one of localized pancreatic tumors [8]. Survival rates vary by location and tumor type, with 5-year overall survival of 80% for P-NENs, 60-100% for localized P-NENs, and 29% for metastatic P-NENs [3, 8]. There are several controversies regarding the influence of size and regional lymph node involvement as poor prognostic factors, however, it has been shown that G3 and the presence of distant metastases are a strong predictor of a poor prognosis [6, 10, 12]. However, even in advanced stages (with the presence of metastases), P-NENs have a better prognosis than pancreatic ductal tumors [5].
The following features the case of a patient who underwent an abdominal surgery in our hospital for presenting a large P-NEN.
2. Case Presentation
We present the case of a 56-year-old patient with a personal history of non-insulin dependent diabetes mellitus, high blood pressure, obesity, deep vein thrombosis, Sd. anxious-depressive reactive to family loss; Sintrom and antihypertensive treatment. No drug allergies were reported. She was admitted to our Hospital’s Internal Medicine Service in October 2015 for bilaterally irradiated epigastric abdominal pain, nausea and dysthermic sensation. She was well hydrated and perfused during Physical examination. A soft, depressible, generally painful abdomen with a large palpable central-abdominal mass was found. A slight anemia and renal failure stood out in the laboratory tests made.
An abdominal Ultrasound was performed, which eported a large neoplasia that seemed to have a retroperitoneal origin, one liver metastasis in segment V and the dilatation of the intrahepatic bile duct. A thoraco-abdominal CT showed a bulky retroperitoneal mass of 158 × 158 × 123 mm that seemed to depend on the uncinate process of pancreas. It had signs of necrosis, mesenteric fat infiltration and multiple gas bubbles in its interior suggesting fistulization with an intestinal loop. An extensive contact with the duodenal framework and the junction of the portal vein and superior mesenteric vein was shown. Mesenteric, peripancreatic, and hepatic hilum lymph nodes greater than 15 mm short axis were described. In liver segment VI, a poorly defined hypodense image of 36 × 22 mm was found, corresponding to the metastasis described in an ultrasound study. The mass produced bile duct dilation (13 mm), slight dilation of the intrahepatic bile duct and Wirsung duct (5 mm). Gastrointestinal stromal tumor (GIST) was suggested as the first option for diagnosis (Figure 1A-1C) and (Figure 2A-2C).
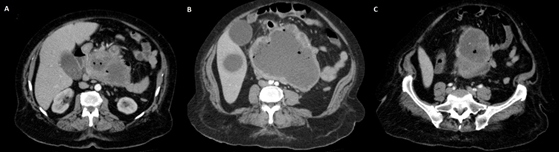
Figure 1A-1C: Axial computed tomography images.
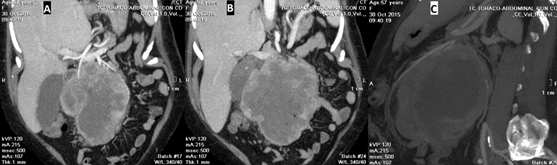
Figure 2A-2C: Coronal (2A, 2B) and sagital (2C) computed tomography images.
In the blood tests made, tumor markers were normal. During admission, the patient presented fever and low gastrointestinal bleeding in the form of melena, so empirical antibiotic treatment as well as the transfusion of 6 red blood cell concentrates were required. Gastroscopy reported suggestive signs of extrinsic compression of duodenum but no sign of mucous infiltration was found. After tumor and liver metastases were biopsied using FNA, the preoperative diagnosis of a moderately differentiated Neuroendocrine tumor (G2) was made.
After the present case was discussed in a Multidisciplinary Tumors Committee, surgery was chosen as the main treatment. Given the progressive worsening of the patient due to sepsis caused by tumor perforation into an intestinal loop, Octreotide Scintigraphy could not be performed. Surgical intervention was carried out in November by a bilateral subcostal access, showing a voluminous 20 cm diameter tumor that depended on the pancreas. Cephalic duodenopancreatectomy was then performed, in addition to a right hemicolectomy because of tumor infiltration of the ascending colon. Two liver space-occupying lesions were confirmed by intraoperative ultrasound, therefore S VI segmentectomy and S IVb metastasectomy were also performed.
On the sixth postoperative day, antibiotic treatment with Piperacillin-Tazobactam was started due to low fever. On the Thirteenth postoperative day, a pancreatic fistula was found but it was correctly directed by drainage, after that, on the fifteenth postoperative day, we evidenced a biliary fistula due to aspiration drainage. Somatostatin intravenous treatment was maintained since the surgical procedure. TPN, antibiotherapy and both drains were gradually withdrawn after performing a control CT showed that the intra-abdominal collections had significantly decreased in size. Thirty four days after surgery the patient was discharged home.
According to pathology (pT3, pN0, m1), the surgical specimen was a 20 × 24 × 5 cm non-functioning G2 neuroendocrine tumor (its Ki67index was between 3% and 20%), belonging to the head of the pancreas. It infiltrated the common bile duct, Vater ampoule and duodenal wall. Surgical resection margins were not affected. No lymphovascular or perineural invasion or limph node metastases were found. IHC profile: Ki67: 18%, synaptophysin positive, focal positive chromogranin and weak positive CKAE1-AE3, CD56 negative. Segmental VI liver resection showed well-differentiated neuroendocrine carcinoma metastasis with extensive necrosis (90%). IVb metastasectomy showed well-differentiated neuroendocrine carcinoma with isolated foci of necrosis. In both cases, free resection margins were reported (Figure 3A, 3B).
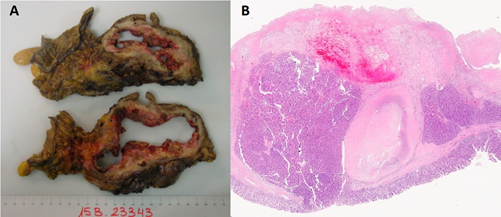
Figure 3A, 3B: Macro (3A) and Micro (3B) Histopathology images.
The patient refused to receive adjuvant treatment. During the follow-up made by the Oncology Service, Serial CT scans, a Somatostatin receptor scyntigraphy, and a SPECT-TC showed disease progression with multiple bilobar liver metastases. Lymph node metastases were also found in the epiphrenic region, lower perigastric region, hiliohepatic, mesentery, and lumboaortic chains. Palliative treatment with analgesia was established. In November 2017, the patient died in the Emergency Department due to cardiorespiratory arrest secondary to pneumonia.
3. Discussion
P-NENs are rare neoplasms (representing 0.46% of gastrointestinal and bronchopulmonary tumors), with a wide spectrum of biological behaviors ranging from benign indolent tumors to very aggressive malignant neoplasms. The prognosis, mortality and overall survival may greatly depend on the tumor characteristics [3, 6, 7, 9]. Due to this wide range of variations, several classifications have been proposed throughout history without achieving some universally accepted validation. WHO 2010 represents the most accepted classification, which separates P-NENs into three different groups (G1, G2 and G3) based on their proliferative index (Ki-67 and mitotic index) [4, 5, 7].
P-NENs are frequently associated with hormonal hypersecretion syndromes. Peptide hormones that can be secreted by these tumors include: insulin, somatostatin, glucagon, pancreatic polypeptide, gastrin, serotonin, calcitonin etc. and they can be analyzed by immunohistochemical studies. Even as Mihalache et al. claim, these tumors can vary from nonfunctioning to functioning tumors throughout their evolution [7]. 90% of P-NENs are sporadic, but they can be associated with some hereditary syndromes, such as multiple endocrine neoplasia type I (MEN1), von Hippel-Lindau (VHL) syndrome, neurofibromatosis type I (NF1), and tuberous sclerosis [1, 2, 7].
The process of diagnosis is not easy, not only because the symptoms of nonfunctioning P-NENs are not specific but also because the recognition of hypersecretion syndromes requires a high index of suspicion. For all these reasons, the diagnosis can be delayed from the onset of symptoms for up to one year [7]. Our case was presented with an evident diagnostic delay since the patient went to the Emergency Department when the tumor was already palpable due to its large size and showed liver metastases.
For Tumor staging and defining the best therapeutic strategy, imaging tests and anatomopathological analysis of biopsy samples were obtained. They confirmed the neuroendcrine origin of the neoplasm and determined its cell proliferation index [1, 7, 9]. Imaging tests such as are Computerized Tomography (CT) and Nuclear Magnetic Resonance (NMR) are recomended not only to measure the tumor but also its invasion of adjacent tissues. CT is known to be less sensitive for detecting pancreatic tumors, but it is more specific than MRI. Regarding liver space-occupying lesions, MRI is the recommended imaging test, since CT has a higher failure rate of metastases detection that could be up to 20% according to Belotto et al. [1]. Somatostatin receptor scintigraphy seems useful for detection of hidden tumors, with 80% sensitivity for G1 and G2 NENs and non-metastatic insulinomas with a high Ki67 index as reported by Mihalache et al. [7]. 68-Ga positron emission tomography (PET)/CT can also vary the therapeutic attitude when detecting small hidden tumors and extrahepatic metastases, except for insulinomas due to its low sensitivity (<20%) [7]. Regarding laboratory tests, the most specific marker for P-NENs is Comogranin A, which is produced by all gastro-pancreatic neuroendocrine tumors. It elevates in 70% of P-NENs (both functioning and nonfunctioning tumors). It should be borne in mind that patients taking regular proton pump inhibitors and those with atrophic gastritis can get false positives [1, 7, 9].
In our reported case, CT and Ultrasound tests were performed. As tumor and liver metastases were both biopsied so a moderately differentiated neuroendocrine tumor (G2) was confirmed, it was not necessary to perform an MRI. It was a nonfunctioning P-NEN since the patient never presented hormonal hypersecretion symptoms. Surgery stands as the only curative treatment depending on tumor size, location, symptoms and presence or absence of metastasis, as well as all the patient's comorbidities [1, 3, 4, 7, 12]. The surgical techniques used are total pancreatectomy, cephalic duodenum pancreatectomy, distal pancreatectomy, tumor enucleation associated with regional lymphadenetomy and metastasectomy and R0 is the objective to be achieved [1, 3, 5]. Recently, several studies have shown that observation may be sufficient for nonfunctioning P-NENs smaller than 2 cm [1, 3, 7], however, other authors argue that despite their small size, these tumors can present aggressive behaviors, which is why they must be considered as malignant tumors from the moment of diagnosis and their surgica removal is recommended [3, 11]. Surgery is also the treatment of choice for metastatic P-NENs, since it has been shown that the primary tumor resection increases the survival rate of these patients [3, 8, 10, 11].
The liver is the most frequent location where the methastatic disease, therefore metastasectomy together with primary tumor resection has been shown to prolong survival rates. However, if the primary tumor is not considered resectable, liver metastases will not be resectable either [3]. For unresectable liver metastases, local therapies such as ablation, chemoembolization or microwaves are recommended by some authors since they seem to improve the prognosis of these patients [7, 11, 12]. Tumors affecting the superior Mesenteric artery, the Celiac trunk or the common Hepatic artery or ocludding the superior Mesenteric vein are considered unresectable at the outset. Treatment with Somatostatin analogues, targeted therapies, chemoembolization, and systemic chemotherapy has been shown to improve and maintain a good quality of life for these non-surgical patients [3, 8, 10].
Even if surgery is the recommended treatment, it is rarely curative without adjuvant treatments. Adjuvant chemotherapy has been shown to improve survival rates in both resected and unresectable tumors, using various drug combinations for this purpose (Cisplatin plus Irinotecan, FOLFIRI or Placlitaxel, Carboplatin plus Etoposide [3]. According to Feng et al. and according to many other previous studies, chemotherapy achieves survival rates of 5.8 to 12 months in unresected NENs [6]. As reflected by Liu et al. patients with P-NENs have long survival rates even if the disease is already at a metastatic stage [3]. According to Deng et al. the overall 5-year survival rate for P-NENs is estimated at 82.6–87.8% for G1, 52.7-70.1% for G2, and 20.7–25.7% for G3 [4]. Darba et al. describe the 5-year overall survival rate as 53.9% in their statistical studies, which can even reach 80% in patients undergoing aggressive resections [5]. But as defended by Feng et al, the survival rating cannot be generalised since it has great variations depending on the location, grade, and treatment of P-NENs As reflected in their systematic review of the literature, the overall survival rates of patients with metastatic P-NENs seemed to be greater in patients undergoing primary tumor resections than in those whom surgery was not performed. Also, overall survival rates were longer in patients with primary tumor resection along with methastasectomy than those patients undergoing just one procedure at a time (only primary tumor resection or methastasectomy) [6]. On the one hand, Tumors located in the pancreatic tail and the uses of chemotherapy are considered to be factors of good prognosis [6]. On the other hand, Derya et al. subscribe non-differentiation (G3) as an independent factor of poor prognosis [8]. According to the univariate and multivariate analysis of Zhang et al., A ki 67 index higher than 10% is an independent P-NENs recurrence factor, therefore, patients presenting these figures must be closely followed in the postoperative period [11].
In our presented case, despite the voluminous size of the primary tumor and the presence of liver methastases, aggressive surgery was performed with curative intent with satisfactory oncological results. However, as adjuvant therapies such as chemotherapy were not added, the survival rate obtained if the treatment had been completed correctly could not be known.
4. Conclusion
Even if P-NENs are rare neoplasms, medical practitioners should be wary of their occurrence and searching for early signs before they present as clearly symptomatic, which would allow us to diagnose them early enough to offer all the wide variety of treatments available with curative intentions in addition to surgery, which seems to be the only curative treatment nowadays. Regarding our case, an aggressive surgery with curative intent was carried out because both tumors (the primary pancreatic tumor and the liver metastases) were considered resectable from the imaging tests, and also because it was a moderately differentiated tumor according to the reported preoperative pathology. Eventually, given the patient's septical progression due to the tumor perforation into an intestinal loop, the surgery had to be carried out in a short period of time; so many other complementary tests were not performed. There are multiple and very complex classifications of NENs but a standarized framework for the classification of NENs is still lacking due to their biological and clinical variability. Therefore, more systematized studies and larger case series are needed.
Competing Interests
The authors declare that they have no competing interests.
References
- Belotto M, Crouzillard BNS, Araujo KO, et al. Pancreatic neuroendocrine tumors: surgical resection. ABCD Arq Bras Cir Dig 32 (2019): e1428.
- Varas-Lorenzo MJ, Cugat E, Capdevila J, et al. Detección de tumores neuroendocrinos pancreáticos: 23 años de experiencia. Rev Gastroenterol Mex 84 (2019): 18-25.
- Liu DJ, Hua R, Sun YW. Current treatment status in pancreatic neuroendocrine neoplasms. Chin Clin Oncol 8 (2019): 20.
- Deng BY, Yang M, Wen JY, et al. Survivals of patients with pancreatic neuroendocrine carcinomas: An in-depth analysis by the American Joint Committee on Cancer 8th tumor-node-metastasis staging manual. Medicine 99 (2020): 3(e18736).
- Darbà J, Marsà A. Exploring the current status of neuroendocrine tumours: a population- based analysis of epidemiology, management and use of resources. BMC Cancer 19 (2019): 1226.
- Feng T, Lv M, Yuan M, et al. Surgical resection of the primary tumor leads to prolonged survival in metastatic pancreatic neuroendocrine carcinoma. World J of Surgical Oncology 17 (2019): 54.
- Mihalache O, Doran H, Poianã C, Bîrligea A, et al. Pancreatic Neuroendocrine Tumors – Case Series and Literature Review. Chirurgia (Bucur) 114 (2019): 630-638.
- Kivrak Salim D, Bayram S, Gömceli I, et al. Palliative resection of primary site in advanced gastroenteropancreatic neuroendocrine tumors improves survivals. Turk J Gastroenterol 30 (2019): 910-916.
- Târcoveanu E, Lupascu C, Vasilescu A, et al. The Pancreatic Endocrine Tumors - Experience of First Surgical Clinic Iasi. Chirurgia (Bucur) 114 (2019): 639-649.
- Wang S, Zhang J, Liu S, et al. The prognostic pattern and involve other distant organs without involving the analysis of different metastatic patterns in pancreatic neuroendocrine tumors liver. These casesmay represent a different subset of patients with patients. Medicine 98 (2019): 44(e17773).
- Zhang P, Li YL, Qiu XD, et al. Clinicopathological characteristics and risk factors for recurrence of well-differentiated pancreatic neuroendocrine tumors after radical surgery: a case-control study. World J Surg Oncol 17 (2019): 66.
- Zhang J, Peng CS, Tian YH. Primary site surgery for elderly patients with distant metastatic pancreatic neuroendocrine tumor: to do or not to do?. Clin Interv Aging 14 (2019): 1419-1432.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
